The fibrinolytic system enables the onset of Plasmodium infection in the mosquito vector and the mammalian host
- PMID: 33547079
- PMCID: PMC7864569
- DOI: 10.1126/sciadv.abe3362
The fibrinolytic system enables the onset of Plasmodium infection in the mosquito vector and the mammalian host
Abstract
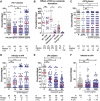
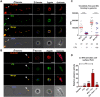
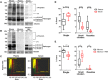
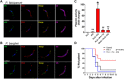
Plasmodium parasites must migrate across proteinaceous matrices to infect the mosquito and vertebrate hosts. Plasmin, a mammalian serine protease, degrades extracellular matrix proteins allowing cell migration through tissues. We report that Plasmodium gametes recruit human plasminogen to their surface where it is processed into plasmin by corecruited plasminogen activators. Inhibition of plasminogen activation arrests parasite development early during sexual reproduction, before ookinete formation. We show that increased fibrinogen and fibrin in the blood bolus, which are natural substrates of plasmin, inversely correlate with parasite infectivity of the mosquito. Furthermore, we show that sporozoites, the parasite form transmitted by the mosquito to humans, also bind plasminogen and plasminogen activators on their surface, where plasminogen is activated into plasmin. Surface-bound plasmin promotes sporozoite transmission by facilitating parasite migration across the extracellular matrices of the dermis and of the liver. The fibrinolytic system is a potential target to hamper Plasmodium transmission.
Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures


References
-
- Wu P., Sun P., Nie K., Zhu Y., Shi M., Xiao C., Liu H., Liu Q., Zhao T., Chen X., Zhou H., Wang P., Cheng G., A gut commensal bacterium promotes mosquito permissiveness to arboviruses. Cell Host Microbe 25, 101–112.e5 (2019). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

