Efficacy and tolerability of bevacizumab in patients with severe Covid-19
- PMID: 33547300
- PMCID: PMC7864918
- DOI: 10.1038/s41467-021-21085-8
Efficacy and tolerability of bevacizumab in patients with severe Covid-19
Abstract
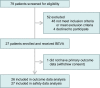
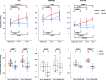
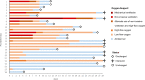
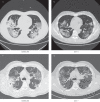
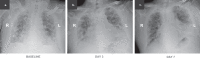
On the basis of Covid-19-induced pulmonary pathological and vascular changes, we hypothesize that the anti-vascular endothelial growth factor (VEGF) drug bevacizumab might be beneficial for treating Covid-19 patients. From Feb 15 to April 5, 2020, we conducted a single-arm trial (NCT04275414) and recruited 26 patients from 2-centers (China and Italy) with severe Covid-19, with respiratory rate ≥30 times/min, oxygen saturation ≤93% with ambient air, or partial arterial oxygen pressure to fraction of inspiration O2 ratio (PaO2/FiO2) >100 mmHg and ≤300 mmHg, and diffuse pneumonia confirmed by chest imaging. Followed up for 28 days. Among these, bevacizumab plus standard care markedly improves the PaO2/FiO2 ratios at days 1 and 7. By day 28, 24 (92%) patients show improvement in oxygen-support status, 17 (65%) patients are discharged, and none show worsen oxygen-support status nor die. Significant reduction of lesion areas/ratios are shown in chest computed tomography (CT) or X-ray within 7 days. Of 14 patients with fever, body temperature normalizes within 72 h in 13 (93%) patients. Relative to comparable controls, bevacizumab shows clinical efficacy by improving oxygenation and shortening oxygen-support duration. Our findings suggest bevacizumab plus standard care is highly beneficial for patients with severe Covid-19. Randomized controlled trial is warranted.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- World Health Organization. Coronavirus disease (Covid-19) outbreak. https://www.who.int (2020).
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

