Domeless receptor loss in fat body tissue reverts insulin resistance induced by a high-sugar diet in Drosophila melanogaster
- PMID: 33547367
- PMCID: PMC7864986
- DOI: 10.1038/s41598-021-82944-4
Domeless receptor loss in fat body tissue reverts insulin resistance induced by a high-sugar diet in Drosophila melanogaster
Abstract
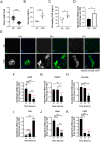
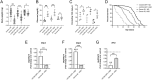
Insulin resistance is a hallmark of type 2 diabetes resulting from the confluence of several factors, including genetic susceptibility, inflammation, and diet. Under this pathophysiological condition, the dysfunction of the adipose tissue triggered by the excess caloric supply promotes the loss of sensitivity to insulin at the local and peripheral level, a process in which different signaling pathways are involved that are part of the metabolic response to the diet. Besides, the dysregulation of insulin signaling is strongly associated with inflammatory processes in which the JAK/STAT pathway plays a central role. To better understand the role of JAK/STAT signaling in the development of insulin resistance, we used a simple organism, Drosophila melanogaster, as a type 2 diabetes model generated by the consumption of a high-sugar diet. In this model, we studied the effects of inhibiting the expression of the JAK/STAT pathway receptor Domeless, in fat body, on adipose metabolism and glycemic control. Our results show that the Domeless receptor loss in fat body cells reverses both hyperglycemia and the increase in the expression of the insulin resistance marker Nlaz, observed in larvae fed a high sugar diet. This effect is consistent with a significant reduction in Dilp2 mRNA expression and an increase in body weight compared to wild-type flies fed high sugar diets. Additionally, the loss of Domeless reduced the accumulation of triglycerides in the fat body cells of larvae fed HSD and also significantly increased the lifespan of adult flies. Taken together, our results show that the loss of Domeless in the fat body reverses at least in part the dysmetabolism induced by a high sugar diet in a Drosophila type 2 diabetes model.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- World Health Organization . Global Report on Diabetes. Geneva: WHO; 2016.
-
- Weiss, M., Steiner, D. F., & Philipson, L. H. Insulin Biosynthesis, Secretion, Structure, and Structure-Activity Relationships (2000). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

