Non-neutralizing antibodies protect against chronic LCMV infection by promoting infection of inflammatory monocytes in mice
- PMID: 33547634
- PMCID: PMC8247883
- DOI: 10.1002/eji.202049068
Non-neutralizing antibodies protect against chronic LCMV infection by promoting infection of inflammatory monocytes in mice
Abstract
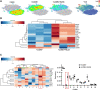
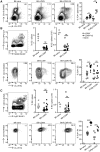
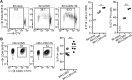
Antibodies play an important role in host defense against microorganisms. Besides direct microbicidal activities, antibodies can also provide indirect protection via crosstalk to constituents of the adaptive immune system. Similar to many human chronic viral infections, persistence of Lymphocytic choriomeningitis virus (LCMV) is associated with compromised T- and B-cell responses. The administration of virus-specific non-neutralizing antibodies (nnAbs) prior to LCMV infection protects against the establishment of chronic infection. Here, we show that LCMV-specific nnAbs bind preferentially Ly6Chi inflammatory monocytes (IMs), promote their infection in an Fc-receptor independent way, and support acquisition of APC properties. By constituting additional T-cell priming opportunities, IMs promote early activation of virus-specific CD8 T cells, eventually tipping the balance between T-cell exhaustion and effector cell differentiation, preventing establishment of viral persistence without causing lethal immunopathology. These results document a beneficial role of IMs in avoiding T-cell exhaustion and an Fc-receptor independent protective mechanism provided by LCMV-specific nnAbs against the establishment of chronic infection.
Keywords: Chronic viral infection; Inflammatory monocytes; LCMV; Non-neutralizing antibodies; T-cell activation.
© 2021 The Authors. European Journal of Immunology published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no commercial or financial conflict of interest.
Figures


References
-
- Schmaljohn, A. L. . Protective antiviral antibodies that precedents and evolution of concepts lack neutralizing activity. Curr. HIV Res. 2013. 11: 345–353. - PubMed
-
- Excler, J. ‐L. , Ake, J. , Robb, M. L. , Kim, J. H. and Plotkin, S. A. . Nonneutralizing functional antibodies: a new ‘old’ paradigm for HIV vaccines. Clin. Vaccine Immunol. 2014. 21: 1–41. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24920599. - PMC - PubMed
-
- Laidlaw, B. J. , Decman, V. , Ali, M.‐.A. A. , Abt, M. C. , Wolf, A. I. , Monticelli, L. A. , Mozdzanowska, K. , et al. Cooperativity between CD8+ T cells, non‐neutralizing antibodies, and alveolar macrophages is important for heterosubtypic influenza virus immunity. PLoS Pathog. 2013. 9: e1003207. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3597515&tool=p... - PMC - PubMed
-
- Wilson, J. A. , Hevey, M. , Bakken, R. , Guest, S. , Bray, M. , Schmaljohn, A. L. and Hart, M. K. . Epitopes involved in antibody‐mediated protection from ebola virus. Science (80). 2000. 287: 1664–1666. Available at: http://www.sciencemag.org/cgi/doi/10.1126/science.287.5458.1664. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

