Biological subphenotypes of acute respiratory distress syndrome may not reflect differences in alveolar inflammation
- PMID: 33547768
- PMCID: PMC7865405
- DOI: 10.14814/phy2.14693
Biological subphenotypes of acute respiratory distress syndrome may not reflect differences in alveolar inflammation
Abstract
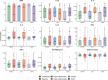
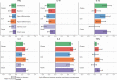
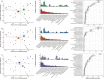
Biological subphenotypes have been identified in acute respiratory distress syndrome (ARDS) based on two parsimonious models: the "uninflamed" and "reactive" subphenotype (cluster-model) and "hypo-inflammatory" and "hyper-inflammatory" (latent class analysis (LCA) model). The distinction between the subphenotypes is mainly driven by inflammatory and coagulation markers in plasma. However, systemic inflammation is not specific for ARDS and it is unknown whether these subphenotypes also reflect differences in the alveolar compartment. Alveolar inflammation and dysbiosis of the lung microbiome have shown to be important mediators in the development of lung injury. This study aimed to determine whether the "reactive" or "hyper-inflammatory" biological subphenotype also had higher concentrations of inflammatory mediators and enrichment of gut-associated bacteria in the lung. Levels of alveolar inflammatory mediators myeloperoxidase (MPO), surfactant protein D (SPD), interleukin (IL)-1b, IL-6, IL-10, IL-8, interferon gamma (IFN-ƴ), and tumor necrosis factor-alpha (TNFα) were determined in the mini-BAL fluid. Key features of the lung microbiome were measured: bacterial burden (16S rRNA gene copies/ml), community diversity (Shannon Diversity Index), and community composition. No statistically significant differences between the "uninflamed" and "reactive" ARDS subphenotypes were found in a selected set of alveolar inflammatory mediators and key features of the lung microbiome. LCA-derived subphenotypes and stratification based on cause of ARDS (direct vs. indirect) showed similar profiles, suggesting that current subphenotypes may not reflect the alveolar host response. It is important for future research to elucidate the pulmonary biology within each subphenotype properly, which is arguably a target for intervention.
© 2021 The Authors. Physiological Reports published by Wiley Periodicals LLC on behalf of The Physiological Society and the American Physiological Society.
Figures
References
-
- Binnie, A. , Tsang, J. L. Y. , & Dos Santos, C. C. (2014). Biomarkers in acute respiratory distress syndrome. Current Opinion in Critical Care, 20(1), 47–55. - PubMed
-
- Bos, L. D. , Schouten, L. R. , van Vught, L. A. , Wiewel, M. A. , Ong, D. S. Y. , Cremer, O. , Artigas, A. , Martin‐Loeches, I. , Hoogendijk, A. J. , van der Poll, T. , Horn, J. , Juffermans, N. , Calfee, C. S. , Schultz, M. J. , & MARS Consortium. (2017). Identification and validation of distinct biological phenotypes in patients with acute respiratory distress syndrome by cluster analysis. Thorax, 72, 876–883. 10.1136/thoraxjnl-2016-209719 - DOI - PMC - PubMed
-
- Calfee, C. S. , Delucchi, K. , Parsons, P. E. , Thompson, B. T. , Ware, L. B. , Matthay, M. A. , & NHLBI ARDS Network. (2014). Subphenotypes in acute respiratory distress syndrome: Latent class analysis of data from two randomised controlled trials. The Lancet Respiratory Medicine, 2(8), 611–620. 10.1016/S2213-2600(14)70097-9 - DOI - PMC - PubMed
-
- Calfee, C. S. , Delucchi, K. L. , Sinha, P. , Matthay, M. A. , Hackett, J. , Shankar‐Hari, M. , McDowell, C. , Laffey, J. G. , O’Kane, C. M. , & McAuley, D. F. , Johnston, A. J. , Paikray, A. , Yates, C. , Polgarova, P. , Price, E. , McInerney, A. , Zamoscik, K. , Dempsey, G. , Seasman, C. , … Sellors, G. (2018). Irish Critical Care Trials Group. Acute respiratory distress syndrome subphenotypes and differential response to simvastatin: Secondary analysis of a randomised controlled trial. The Lancet Respiratory Medicine, 6, 691–698. 10.1016/S2213-2600(18)30177-2 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

