Type I SMA "new natural history": long-term data in nusinersen-treated patients
- PMID: 33547876
- PMCID: PMC7951096
- DOI: 10.1002/acn3.51276
Type I SMA "new natural history": long-term data in nusinersen-treated patients
Abstract
Objective: The aim of this paper was to report the 2-year follow-up in type I patients treated with Nusinersen and to assess whether possible changes in motor function are related to the subtype, age, or SMN2 copy number.
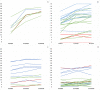
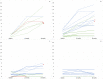
Methods: Sixty-eight patients, with ages ranging from 0.20 to 15.92 years (mean: 3.96; standard deviation: +3.90) were enrolled in the study. All patients were assessed using the Children's Hospital of Philadelphia Infant Test of Neuromuscular Disorders (CHOP INTEND) and the developmental section of the Hammersmith Infant Neurological Examination (HINE-2) at the time they started treatment and 12 and 24 months after that.
Results: For both CHOP and HINE-2 repeated measures analysis of variance showed a significant difference (P < 0.001) between baseline and 12 months, 12 months and 24 months, and baseline and 24-month scores for the whole group. When age subgroups (<210 days, <2 years, 2-4 years, 5-11 years, 12-18 years) were considered, on the CHOP INTEND the difference was significant between baseline and 24 months in all age subgroups. On the HINE-2, the difference between baseline and 24 months was significant in all the subgroups before the age of 4 years. Age was predictive of changes on both scales (P < 0.05), whereas SMN2 copy number and decimal classification were not.
Interpretation: Our results suggest that some improvement of motor function can be observed even after the first year of treatment. This is more obvious in the infants treated in the first 2 years but some improvement can also be found in older children.
© 2021 The Authors. Annals of Clinical and Translational Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
GC, RDS, MP, SM, ADA, EB, VAS, CB, and EM has been a consultant for BIOGEN S.R.L. which owns patent rights to nusinersen that was used in this study.
Figures


References
-
- Aragon‐Gawinska K, Daron A, Ulinici A, et al. Sitting in patients with spinal muscular atrophy type 1 treated with nusinersen. Dev Med Child Neurol 2020;62:310–314. - PubMed
-
- Aragon‐Gawinska K, Seferian AM, Daron A, et al. Nusinersen in patients older than 7 months with spinal muscular atrophy type 1: a cohort study. Neurology 2018;91:e1312–e1318. - PubMed
-
- Pechmann A, Langer T, Wider S, Kirschner J. Single‐center experience with intrathecal administration of Nusinersen in children with spinal muscular atrophy type 1. Eur J Paediatr Neurol 2018;22:122–127. - PubMed
-
- Pane M, Coratti G, Sansone VA, et al. Nusinersen in type 1 spinal muscular atrophy: twelve‐month real‐world data. Ann Neurol 2019;86:443–451. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

