Chromosome Y pericentric heterochromatin is a primary target of HSF1 in male cells
- PMID: 33547955
- PMCID: PMC7889540
- DOI: 10.1007/s00412-021-00751-2
Chromosome Y pericentric heterochromatin is a primary target of HSF1 in male cells
Abstract
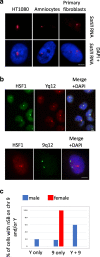
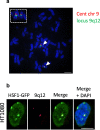
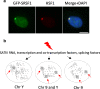
The heat shock factor 1 (HSF1)-dependent transcriptional activation of human pericentric heterochromatin in heat-shocked cells is the most striking example of transcriptional activation of heterochromatin. Until now, pericentric heterochromatin of chromosome 9 has been identified as the primary target of HSF1, in both normal and tumor heat-shocked cells. Transcriptional awakening of this large genomic region results in the nuclear accumulation of satellite III (SATIII) noncoding RNAs (ncRNAs) and the formation in cis of specific structures known as nuclear stress bodies (nSBs). Here, we show that, in four different male cell lines, including primary human fibroblasts and amniocytes, pericentric heterochromatin of chromosome Y can also serve as a unique primary site of HSF1-dependent heterochromatin transcriptional activation, production of SATIII ncRNA, and nucleation of nuclear stress bodies (nSBs) upon heat shock. Our observation suggests that the chromosomal origin of SATIII transcripts in cells submitted to heat shock is not a determinant factor as such, but that transcription of SATIII repetitive units or the SATIII ncRNA molecules is the critical element of HSF1-dependent transcription activation of constitutive heterochromatin.
Keywords: HSF1; Heterochromatin; Human; nSB; ncRNA.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

