Projections from D2 Neurons in Different Subregions of Nucleus Accumbens Shell to Ventral Pallidum Play Distinct Roles in Reward and Aversion
- PMID: 33548029
- PMCID: PMC8099949
- DOI: 10.1007/s12264-021-00632-9
Projections from D2 Neurons in Different Subregions of Nucleus Accumbens Shell to Ventral Pallidum Play Distinct Roles in Reward and Aversion
Abstract
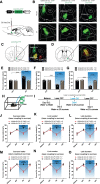
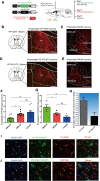
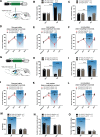
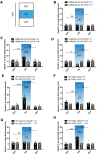
The nucleus accumbens shell (NAcSh) plays an important role in reward and aversion. Traditionally, NAc dopamine receptor 2-expressing (D2) neurons are assumed to function in aversion. However, this has been challenged by recent reports which attribute positive motivational roles to D2 neurons. Using optogenetics and multiple behavioral tasks, we found that activation of D2 neurons in the dorsomedial NAcSh drives preference and increases the motivation for rewards, whereas activation of ventral NAcSh D2 neurons induces aversion. Stimulation of D2 neurons in the ventromedial NAcSh increases movement speed and stimulation of D2 neurons in the ventrolateral NAcSh decreases movement speed. Combining retrograde tracing and in situ hybridization, we demonstrated that glutamatergic and GABAergic neurons in the ventral pallidum receive inputs differentially from the dorsomedial and ventral NAcSh. All together, these findings shed light on the controversy regarding the function of NAcSh D2 neurons, and provide new insights into understanding the heterogeneity of the NAcSh.
Keywords: Aversion; D2 neurons; Motivation; Nucleus accumbens shell; Reward; Ventral pallidum.
Conflict of interest statement
All authors claim that there are no conflict of interest.
Figures


References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

