Adenosine A1 and A2A receptors are involved on guanosine protective effects against oxidative burst and mitochondrial dysfunction induced by 6-OHDA in striatal slices
- PMID: 33548045
- PMCID: PMC8155135
- DOI: 10.1007/s11302-021-09765-y
Adenosine A1 and A2A receptors are involved on guanosine protective effects against oxidative burst and mitochondrial dysfunction induced by 6-OHDA in striatal slices
Abstract
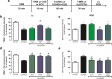
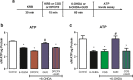
6-Hydroxydopamine (6-OHDA) is the most used toxin in experimental Parkinson's disease (PD) models. 6-OHDA shows high affinity for the dopamine transporter and once inside the neuron, it accumulates and undergoes non-enzymatic auto-oxidation, promoting reactive oxygen species (ROS) formation and selective damage of catecholaminergic neurons. In this way, our group has established a 6-OHDA in vitro protocol with rat striatal slices as a rapid and effective model for screening of new drugs with protective effects against PD. We have shown that co-incubation with guanosine (GUO, 100 μM) prevented the 6-OHDA-induced damage in striatal slices. As the exact GUO mechanism of action remains unknown, the aim of this study was to investigate if adenosine A1 (A1R) and/or A2A receptors (A2AR) are involved on GUO protective effects on striatal slices. Pre-incubation with DPCPX, an A1R antagonist prevented guanosine effects on 6-OHDA-induced ROS formation and mitochondrial membrane potential depolarization, while CCPA, an A1R agonist, did not alter GUO effects. Regarding A2AR, the antagonist SCH58261 had similar protective effect as GUO in ROS formation and mitochondrial membrane potential. Additionally, SCH58261 did not affect GUO protective effects. The A2AR agonist CGS21680, although, completely blocked GUO effects. Finally, the A1R antagonist DPCPX, and the A2AR agonist CGS21680 also abolished the preventive guanosine effect on 6-OHDA-induced ATP levels decrease. These results reinforce previous evidence for a putative interaction of GUO with A1R-A2AR heteromer as its molecular target and clearly indicate a dependence on adenosine receptors modulation to GUO protective effect.
Keywords: 6-Hydroxydopamine, Striatal slices; Adenosine A1 and A2A receptors; Guanosine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Involvement of adenosine A1 and A2A receptors on guanosine-mediated anti-tremor effects in reserpinized mice.Purinergic Signal. 2020 Sep;16(3):379-387. doi: 10.1007/s11302-020-09716-z. Epub 2020 Jul 28. Purinergic Signal. 2020. PMID: 32725400 Free PMC article.
-
Guanosine Protects Striatal Slices Against 6-OHDA-Induced Oxidative Damage, Mitochondrial Dysfunction, and ATP Depletion.Neurotox Res. 2019 Feb;35(2):475-483. doi: 10.1007/s12640-018-9976-1. Epub 2018 Nov 12. Neurotox Res. 2019. PMID: 30417317
-
Guanosine-Mediated Anxiolytic-Like Effect: Interplay with Adenosine A1 and A2A Receptors.Int J Mol Sci. 2020 Dec 5;21(23):9281. doi: 10.3390/ijms21239281. Int J Mol Sci. 2020. PMID: 33291390 Free PMC article.
-
Adenosine A1 and A2A Receptors in the Brain: Current Research and Their Role in Neurodegeneration.Molecules. 2017 Apr 23;22(4):676. doi: 10.3390/molecules22040676. Molecules. 2017. PMID: 28441750 Free PMC article. Review.
-
Functional and Neuroprotective Role of Striatal Adenosine A2A Receptor Heterotetramers.J Caffeine Adenosine Res. 2019 Sep 1;9(3):89-97. doi: 10.1089/caff.2019.0008. Epub 2019 Sep 17. J Caffeine Adenosine Res. 2019. PMID: 31559390 Free PMC article. Review.
Cited by
-
Adenosinergic Pathway in Parkinson's Disease: Recent Advances and Therapeutic Perspective.Mol Neurobiol. 2023 Jun;60(6):3054-3070. doi: 10.1007/s12035-023-03257-3. Epub 2023 Feb 14. Mol Neurobiol. 2023. PMID: 36786912 Review.
-
Dynamic and Rapid Detection of Guanosine during Ischemia.ACS Chem Neurosci. 2023 May 3;14(9):1646-1658. doi: 10.1021/acschemneuro.3c00048. Epub 2023 Apr 11. ACS Chem Neurosci. 2023. PMID: 37040534 Free PMC article.
-
Neuroprotection afforded by targeting G protein-coupled receptors in heteromers and by heteromer-selective drugs.Front Pharmacol. 2023 Jul 13;14:1222158. doi: 10.3389/fphar.2023.1222158. eCollection 2023. Front Pharmacol. 2023. PMID: 37521478 Free PMC article. Review.
-
Lessons from the physiological role of guanosine in neurodegeneration and cancer: Toward a multimodal mechanism of action?Purinergic Signal. 2025 Feb;21(1):133-148. doi: 10.1007/s11302-024-10033-y. Epub 2024 Jul 15. Purinergic Signal. 2025. PMID: 39004650 Free PMC article. Review.
-
Ginsenoside Rg1 in Parkinson's disease: from basic research to clinical applications.Front Pharmacol. 2025 Apr 16;16:1490480. doi: 10.3389/fphar.2025.1490480. eCollection 2025. Front Pharmacol. 2025. PMID: 40308780 Free PMC article. Review.
References
-
- Pereira D, Garrett C. Risk factors for Parkinson disease: an epidemiologic study. Acta Medica Port. 2010;23(1):15–24. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

