Gating of hippocampal rhythms and memory by synaptic plasticity in inhibitory interneurons
- PMID: 33548174
- PMCID: PMC9239733
- DOI: 10.1016/j.neuron.2021.01.014
Gating of hippocampal rhythms and memory by synaptic plasticity in inhibitory interneurons
Abstract
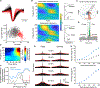
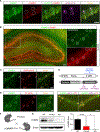
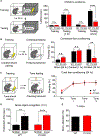
Mental experiences can become long-term memories if the hippocampal activity patterns that encode them are broadcast during network oscillations. The activity of inhibitory neurons is essential for generating these neural oscillations, but molecular control of this dynamic process during learning remains unknown. Here, we show that hippocampal oscillatory strength positively correlates with excitatory monosynaptic drive onto inhibitory neurons (E→I) in freely behaving mice. To establish a causal relationship between them, we identified γCaMKII as the long-sought mediator of long-term potentiation for E→I synapses (LTPE→I), which enabled the genetic manipulation of experience-dependent E→I synaptic input/plasticity. Deleting γCaMKII in parvalbumin interneurons selectively eliminated LTPE→I and disrupted experience-driven strengthening in theta and gamma rhythmicity. Behaviorally, this manipulation impaired long-term memory, for which the kinase activity of γCaMKII was required. Taken together, our data suggest that E→I synaptic plasticity, exemplified by LTPE→I, plays a gatekeeping role in tuning experience-dependent brain rhythms and mnemonic function.
Keywords: CaMKII; LTP; inhibitory interneurons; learning and memory; network oscillations; network plasticity; synaptic plasticity.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests H.H. and S.D. are members of advisory boards for the journal Neuron.
Figures




References
-
- Allen K, and Monyer H. (2015). Interneuron control of hippocampal oscillations. Curr. Opin. Neurobiol. 31, 81–87. - PubMed
-
- Bartos M, Vida I, and Jonas P. (2007). Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat. Rev. Neurosci. 8, 45–56. - PubMed
-
- Bliss TV, and Collingridge GL (1993). A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361, 31–39. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

