Drosophila female germline stem cells undergo mitosis without nuclear breakdown
- PMID: 33548191
- PMCID: PMC8044017
- DOI: 10.1016/j.cub.2021.01.033
Drosophila female germline stem cells undergo mitosis without nuclear breakdown
Abstract
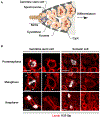
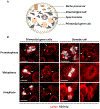
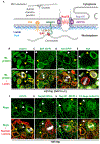
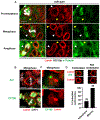
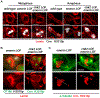
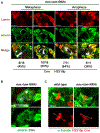
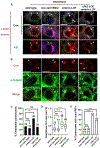
Stem cell homeostasis requires nuclear lamina (NL) integrity. In Drosophila germ cells, compromised NL integrity activates the ataxia telangiectasia and Rad3-related (ATR) and checkpoint kinase 2 (Chk2) checkpoint kinases, blocking germ cell differentiation and causing germline stem cell (GSC) loss. Checkpoint activation occurs upon loss of either the NL protein emerin or its partner barrier-to-autointegration factor, two proteins required for nuclear reassembly at the end of mitosis. Here, we examined how mitosis contributes to NL structural defects linked to checkpoint activation. These analyses led to the unexpected discovery that wild-type female GSCs utilize a non-canonical mode of mitosis, one that retains a permeable but intact nuclear envelope and NL. We show that the interphase NL is remodeled during mitosis for insertion of centrosomes that nucleate the mitotic spindle within the confines of the nucleus. We show that depletion or loss of NL components causes mitotic defects, including compromised chromosome segregation associated with altered centrosome positioning and structure. Further, in emerin mutant GSCs, centrosomes remain embedded in the interphase NL. Notably, these embedded centrosomes carry large amounts of pericentriolar material and nucleate astral microtubules, revealing a role for emerin in the regulation of centrosome structure. Epistasis studies demonstrate that defects in centrosome structure are upstream of checkpoint activation, suggesting that these centrosome defects might trigger checkpoint activation and GSC loss. Connections between NL proteins and centrosome function have implications for mechanisms associated with NL dysfunction in other stem cell populations, including NL-associated diseases, such as laminopathies.
Keywords: Drosophila oogenesis; LEM-domain proteins; barrier-to-autointegration factor; centrosome; checkpoint kinase 2; emerin; germline stem cells; mitosis; nuclear lamina; otefin.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures
References
-
- Gruenbaum Y, Margalit A, Goldman RD, Shumaker DK, and Wilson KL (2005). The nuclear lamina comes of age. Nat Rev Mol Cell Biol 6, 21–31. - PubMed
-
- Goldman RD, Gruenbaum Y, Moir RD, Shumaker DK, and Spann TP (2002). Nuclear lamins: building blocks of nuclear architecture. Genes Dev 16, 533–547. - PubMed
-
- Burke B, and Stewart CL (2014). Functional architecture of the cell’s nucleus in development, aging, and disease. Curr Top Dev Biol 109, 1–52. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

