Hepatic LDL receptor-related protein-1 deficiency alters mitochondrial dynamics through phosphatidylinositol 4,5-bisphosphate reduction
- PMID: 33548224
- PMCID: PMC7949165
- DOI: 10.1016/j.jbc.2021.100370
Hepatic LDL receptor-related protein-1 deficiency alters mitochondrial dynamics through phosphatidylinositol 4,5-bisphosphate reduction
Abstract
The LDL receptor-related protein 1 (LRP1) is a multifunctional transmembrane protein with endocytosis and signal transduction functions. Previous studies have shown that hepatic LRP1 deficiency exacerbates diet-induced steatohepatitis and insulin resistance via mechanisms related to increased lysosome and mitochondria permeability and dysfunction. The current study examined the impact of LRP1 deficiency on mitochondrial function in the liver. Hepatocytes isolated from liver-specific LRP1 knockout (hLrp1-/-) mice showed reduced oxygen consumption compared with control mouse hepatocytes. The mitochondria in hLrp1-/- mouse livers have an abnormal morphology and their membranes contain significantly less anionic phospholipids, including lower levels of phosphatidylethanolamine and cardiolipin that increase mitochondrial fission and impair fusion. Additional studies showed that LRP1 complexes with phosphatidylinositol 4-phosphate 5-kinase like protein-1 (PIP5KL1) and phosphatidylinositol 4-phosphate 5-kinase-1β (PIP5K1β). The absence of LRP1 reduces the levels of both PIP5KL1 and PIP5K1β in the plasma membrane and also lowers phosphatidylinositol(4,5) bisphosphate (PI(4,5)P2) levels in hepatocytes. These data indicate that LRP1 recruits PIP5KL1 and PIP5K1β to the plasma membrane for PI(4,5)P2 biosynthesis. The lack of LRP1 reduces lipid kinase expression, leading to lower PI(4,5)P2 levels, thereby decreasing the availability of this lipid metabolite in the cardiolipin biosynthesis pathway to cause cardiolipin reduction and the impairment in mitochondria homeostasis. Taken together, the current study identifies another signaling mechanism by which LRP1 regulates cell functions: binding and recruitment of PIP5KL1 and PIP5K1β to the membrane for PI(4,5)P2 synthesis. In addition, it highlights the importance of this mechanism for maintaining the integrity and functions of intracellular organelles.
Keywords: cardiolipin; inositol phosphate; lipoprotein receptor; lipoprotein receptor-related protein (LRP); liver metabolism; respiration.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures







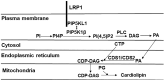
Similar articles
-
LRP1 Protein Deficiency Exacerbates Palmitate-induced Steatosis and Toxicity in Hepatocytes.J Biol Chem. 2016 Aug 5;291(32):16610-9. doi: 10.1074/jbc.M116.717744. Epub 2016 Jun 17. J Biol Chem. 2016. PMID: 27317662 Free PMC article.
-
Hepatic deficiency of low density lipoprotein receptor-related protein-1 reduces high density lipoprotein secretion and plasma levels in mice.J Biol Chem. 2011 Apr 15;286(15):13079-87. doi: 10.1074/jbc.M111.229369. Epub 2011 Feb 22. J Biol Chem. 2011. PMID: 21343303 Free PMC article.
-
Low-density lipoprotein receptor-related protein-1 dysfunction synergizes with dietary cholesterol to accelerate steatohepatitis progression.J Biol Chem. 2018 Jun 22;293(25):9674-9684. doi: 10.1074/jbc.RA118.001952. Epub 2018 May 11. J Biol Chem. 2018. PMID: 29752404 Free PMC article.
-
LDL receptor-related protein 1 and its interacting partners in tissue homeostasis.Curr Opin Lipidol. 2021 Oct 1;32(5):301-307. doi: 10.1097/MOL.0000000000000776. Curr Opin Lipidol. 2021. PMID: 34310383 Free PMC article. Review.
-
News on the molecular regulation and function of hepatic low-density lipoprotein receptor and LDLR-related protein 1.Curr Opin Lipidol. 2017 Jun;28(3):241-247. doi: 10.1097/MOL.0000000000000411. Curr Opin Lipidol. 2017. PMID: 28301372 Free PMC article. Review.
Cited by
-
Convergent molecular evolution of thermogenesis and circadian rhythm in Arctic ruminants.Proc Biol Sci. 2023 May 31;290(1999):20230538. doi: 10.1098/rspb.2023.0538. Epub 2023 May 31. Proc Biol Sci. 2023. PMID: 37253422 Free PMC article.
References
-
- Llorente-Cortes V., Royo T., Juan-Babot O., Badimon L. Adipocyte differentiation-related protein is induced by LRP1-mediated aggregated LDL internalization in human vascular smooth muscle cells and macrophages. J. Lipid Res. 2007;48:2133–2140. - PubMed
-
- Luo L., Wall A.A., Tong S.J., Hung Y., Xiao Z., Tarique A.A., Sly P.D., Fantino E., Marzolo M.P., Stow J.L. TLR crosstalk activates LRP1 to recruit Rab8a and PI3Kγ for suppression of inflammatory responses. Cell Rep. 2018;24:3033–3044. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

