Exosome-based liquid biopsies in cancer: opportunities and challenges
- PMID: 33548389
- PMCID: PMC8268076
- DOI: 10.1016/j.annonc.2021.01.074
Exosome-based liquid biopsies in cancer: opportunities and challenges
Abstract
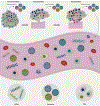
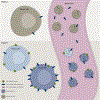
Liquid biopsy in cancer has gained momentum in clinical research and is experiencing a boom for a variety of applications. There are significant efforts to utilize liquid biopsies in cancer for early detection and treatment stratification, as well as residual disease and recurrence monitoring. Although most efforts have used circulating tumor cells and circulating tumor DNA for this purpose, exosomes and other extracellular vesicles have emerged as a platform with potentially broader and complementary applications. Exosomes/extracellular vesicles are small vesicles released by cells, including cancer cells, into the surrounding biofluids. These exosomes contain tumor-derived materials such as DNA, RNA, protein, lipid, sugar structures, and metabolites. In addition, exosomes carry molecules on their surface that provides clues regarding their origin, making it possible to sort vesicle types and enrich signatures from tissue-specific origins. Exosomes are part of the intercellular communication system and cancer cells frequently use them as biological messengers to benefit their growth. Since exosomes are part of the disease process, they have become of tremendous interest in biomarker research. Exosomes are remarkably stable in biofluids, such as plasma and urine, and can be isolated for clinical evaluation even in the early stages of the disease. Exosome-based biomarkers have quickly become adopted in the clinical arena and the first exosome RNA-based prostate cancer test has already helped >50 000 patients in their decision process and is now included in the National Comprehensive Cancer Network guidelines for early prostate cancer detection. This review will discuss the advantages and challenges of exosome-based liquid biopsies for tumor biomarkers and clinical implementation in the context of circulating tumor DNA and circulating tumor cells.
Keywords: cell-free DNA (cfDNA); circulating tumor DNA (ctDNA); circulating tumor cell (CTC); exosome; extracellular vesicle; liquid biopsy.
Copyright © 2021 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Disclosure WY, JH, DR, SC, DE, MN, and JS are employees of Exosome Diagnostics, a Bio-Techne brand.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

