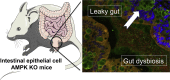
Deletion of intestinal epithelial AMP-activated protein kinase alters distal colon permeability but not glucose homeostasis
- PMID: 33548500
- PMCID: PMC7921883
- DOI: 10.1016/j.molmet.2021.101183
Deletion of intestinal epithelial AMP-activated protein kinase alters distal colon permeability but not glucose homeostasis
Abstract
Objective: The intestinal epithelial barrier (IEB) restricts the passage of microbes and potentially harmful substances from the lumen through the paracellular space, and rupture of its integrity is associated with a variety of gastrointestinal disorders and extra-digestive diseases. Increased IEB permeability has been linked to disruption of metabolic homeostasis leading to obesity and type 2 diabetes. Interestingly, recent studies have uncovered compelling evidence that the AMP-activated protein kinase (AMPK) signaling pathway plays an important role in maintaining epithelial cell barrier function. However, our understanding of the function of intestinal AMPK in regulating IEB and glucose homeostasis remains sparse.
Methods: We generated mice lacking the two α1 and α2 AMPK catalytic subunits specifically in intestinal epithelial cells (IEC AMPK KO) and determined the physiological consequences of intestinal-specific deletion of AMPK in response to high-fat diet (HFD)-induced obesity. We combined histological, functional, and integrative analyses to ascertain the effects of gut AMPK loss on intestinal permeability in vivo and ex vivo and on the development of obesity and metabolic dysfunction. We also determined the impact of intestinal AMPK deletion in an inducible mouse model (i-IEC AMPK KO) by measuring IEB function, glucose homeostasis, and the composition of gut microbiota via fecal 16S rRNA sequencing.
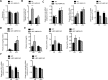
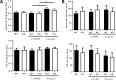
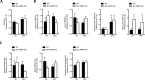
Results: While there were no differences in in vivo intestinal permeability in WT and IEC AMPK KO mice, ex vivo transcellular and paracellular permeability measured in Ussing chambers was significantly increased in the distal colon of IEC AMPK KO mice. This was associated with a reduction in pSer425 GIV phosphorylation, a marker of leaky gut barrier. However, the expression of tight junction proteins in intestinal epithelial cells and pro-inflammatory cytokines in the lamina propria were not different between genotypes. Although the HFD-fed AMPK KO mice displayed suppression of the stress polarity signaling pathway and a concomitant increase in colon permeability, loss of intestinal AMPK did not exacerbate body weight gain or adiposity. Deletion of AMPK was also not sufficient to alter glucose homeostasis or the acute glucose-lowering action of metformin in control diet (CD)- or HFD-fed mice. CD-fed i-IEC AMPK KO mice also presented higher permeability in the distal colon under homeostatic conditions but, surprisingly, this was not detected upon HFD feeding. Alteration in epithelial barrier function in the i-IEC AMPK KO mice was associated with a shift in the gut microbiota composition with higher levels of Clostridiales and Desulfovibrionales.
Conclusions: Altogether, our results revealed a significant role of intestinal AMPK in maintaining IEB integrity in the distal colon but not in regulating glucose homeostasis. Our data also highlight the complex interaction between gut microbiota and host AMPK.
Keywords: AMPK; Intestinal epithelial barrier (IEB); Metformin; Microbiota; Obesity; Permeability.
Copyright © 2021 The Author(s). Published by Elsevier GmbH.. All rights reserved.
Figures












Similar articles
-
Intestinal Epithelial AMPK Deficiency Causes Delayed Colonic Epithelial Repair in DSS-Induced Colitis.Cells. 2022 Feb 9;11(4):590. doi: 10.3390/cells11040590. Cells. 2022. PMID: 35203241 Free PMC article.
-
AMPK improves gut epithelial differentiation and barrier function via regulating Cdx2 expression.Cell Death Differ. 2017 May;24(5):819-831. doi: 10.1038/cdd.2017.14. Epub 2017 Feb 24. Cell Death Differ. 2017. PMID: 28234358 Free PMC article.
-
Neurotensin inhibits AMPK activity and concurrently enhances FABP1 expression in small intestinal epithelial cells associated with obesity and aging.Exp Mol Med. 2025 Jun;57(6):1189-1201. doi: 10.1038/s12276-025-01461-w. Epub 2025 Jun 2. Exp Mol Med. 2025. PMID: 40451927 Free PMC article.
-
Mitochondrial function in intestinal epithelium homeostasis and modulation in diet-induced obesity.Mol Metab. 2022 Sep;63:101546. doi: 10.1016/j.molmet.2022.101546. Epub 2022 Jul 8. Mol Metab. 2022. PMID: 35817394 Free PMC article. Review.
-
Negative Effects of a High-Fat Diet on Intestinal Permeability: A Review.Adv Nutr. 2020 Jan 1;11(1):77-91. doi: 10.1093/advances/nmz061. Adv Nutr. 2020. PMID: 31268137 Free PMC article. Review.
Cited by
-
Low-dose metformin targets the lysosomal AMPK pathway through PEN2.Nature. 2022 Mar;603(7899):159-165. doi: 10.1038/s41586-022-04431-8. Epub 2022 Feb 23. Nature. 2022. PMID: 35197629 Free PMC article.
-
Anti-Hyperglycemic Agents in the Adjuvant Treatment of Sepsis: Improving Intestinal Barrier Function.Drug Des Devel Ther. 2022 Jun 4;16:1697-1711. doi: 10.2147/DDDT.S360348. eCollection 2022. Drug Des Devel Ther. 2022. PMID: 35693534 Free PMC article. Review.
-
Metformin: The Winding Path from Understanding Its Molecular Mechanisms to Proving Therapeutic Benefits in Neurodegenerative Disorders.Pharmaceuticals (Basel). 2023 Dec 11;16(12):1714. doi: 10.3390/ph16121714. Pharmaceuticals (Basel). 2023. PMID: 38139841 Free PMC article. Review.
-
Metformin: Diverse molecular mechanisms, gastrointestinal effects and overcoming intolerance in type 2 Diabetes Mellitus: A review.Medicine (Baltimore). 2024 Oct 25;103(43):e40221. doi: 10.1097/MD.0000000000040221. Medicine (Baltimore). 2024. PMID: 39470509 Free PMC article. Review.
-
Hydroxytyrosol Alleviates Intestinal Oxidative Stress by Regulating Bile Acid Metabolism in a Piglet Model.Int J Mol Sci. 2024 May 21;25(11):5590. doi: 10.3390/ijms25115590. Int J Mol Sci. 2024. PMID: 38891778 Free PMC article.
References
-
- Chang J., Leong R.W., Wasinger V.C., Ip M., Yang M., Phan T.G. Impaired intestinal permeability contributes to ongoing bowel symptoms in patients with inflammatory bowel disease and mucosal healing. Gastroenterology. 2017;153(3):723–731. e721. - PubMed
-
- Vivinus-Nebot M., Frin-Mathy G., Bzioueche H., Dainese R., Bernard G., Anty R. Functional bowel symptoms in quiescent inflammatory bowel diseases: role of epithelial barrier disruption and low-grade inflammation. Gut. 2014;63(5):744–752. - PubMed
-
- Wyatt J., Vogelsang H., Hubl W., Waldhoer T., Lochs H. Intestinal permeability and the prediction of relapse in Crohn's disease. Lancet. 1993;341(8858):1437–1439. - PubMed
-
- Leech B., McIntyre E., Steel A., Sibbritt D. Risk factors associated with intestinal permeability in an adult population: a systematic review. International Journal of Clinical Practice. 2019;73(10) - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

