Signaling pathways of EBV-induced oncogenesis
- PMID: 33549103
- PMCID: PMC7868022
- DOI: 10.1186/s12935-021-01793-3
Signaling pathways of EBV-induced oncogenesis
Abstract
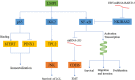
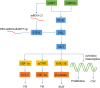
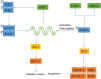
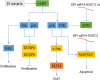
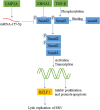
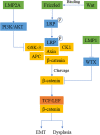
Epstein-Barr virus (EBV) is closely associated with multiple human cancers. EBV-associated cancers are mainly lymphomas derived from B cells and T cells (Hodgkin lymphoma, Burkitt lymphoma, NK/T-cell lymphoma, and posttransplant lymphoproliferative disorder (PTLD)) and carcinomas derived from epithelial cells (nasopharyngeal carcinoma and gastric carcinoma). EBV can induce oncogenesis in its host cell by activating various signaling pathways, such as nuclear factor-κB (NF-κB), phosphoinositide-3-kinase/protein kinase B (PI3K/AKT), Janus kinase/signal transducer and transcription activator (JAK/STAT), mitogen-activated protein kinase (MAPK), transforming growth factor-β (TGF-β), and Wnt/β-catenin, which are regulated by EBV-encoded proteins and noncoding RNA. In this review, we focus on the oncogenic roles of EBV that are mediated through the aforementioned signaling pathways.
Keywords: Cancer; Epstein‐barr virus (EBV); Signaling pathway.
Conflict of interest statement
Authors declare no conflicts of interest for this article.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

