Detecting the Dark Matter of Unpublished Clinical Cancer Studies: An Analysis of Phase 3 Randomized Controlled Trials
- PMID: 33549260
- PMCID: PMC12033004
- DOI: 10.1016/j.mayocp.2020.08.015
Detecting the Dark Matter of Unpublished Clinical Cancer Studies: An Analysis of Phase 3 Randomized Controlled Trials
Abstract
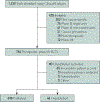
Unpublished randomized controlled trial (RCT) frequency, correlates, and financial impact are not well understood. We sought to characterize the nonpublication of peer-reviewed manuscripts among interventional, therapeutic, multi-arm, phase 3 oncology RCTs. Trials were identified by searching ClinicalTrials.gov, while publications and abstracts were identified through PubMed and Google Scholar. Trial data were extracted from ClinicalTrials.gov and individual publications. Publication was defined as a peer-reviewed manuscript addressing the primary endpoint. Patient accrual cost was extrapolated from experimental data; investigators/sponsors were contacted to determine nonpublication reasons. Six hundred eighty-four completed RCTs met inclusion criteria, which accrued 434,610 patients from 1994 to 2015; 638 were published (93.3%) and 46 were unpublished (6.7%). Among the unpublished trials, the time difference from primary endpoint maturity to data abstraction was a median of 6 years (interquartile range, 4 to 8 years). On multiple binary logistic regression analysis, factors associated with unpublished trials included lack of cooperative group sponsorship (odds ratio, 5.91, 95% CI, 1.35 to 25.97; P=.019) and supportive care investigation (odds ratio, 2.90; 95% CI, 1.13 to 7.41; P=.027). The estimated inflation-adjusted average cost of patient accrual for all unpublished trials was $113,937,849 (range, $41,136,883 to $320,201,063). Direct contact with sponsors/investigators led to a 50.0% response rate (n=23 of 46); manuscript in preparation and/or in submission (n=10 of 23) was the most commonly cited reason for nonpublication. In conclusion, approximately 1 in 15 clinical oncology RCTs are unpublished and this has a profound impact on the research enterprise. The cooperative group infrastructure may serve as a blueprint to reduce nonpublication.
Copyright © 2020 Mayo Foundation for Medical Education and Research. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures
References
-
- World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. - PubMed
-
- Turner EH, Matthews AM, Linardatos E, Tell RA, Rosenthal R. Selective publication of antidepressant trials and its influence on apparent efficacy. N Engl J Med. 2008;358(3):252–260. - PubMed
-
- Whittington CJ, Kendall T, Fonagy P, Cottrell D, Cotgrove A, Boddington E. Selective serotonin reuptake inhibitors in childhood depression: systematic review of published versus unpublished data. Lancet. 2004;363(9418):1341–1345. - PubMed
-
- Roddick AJ, Chan FTS, Stefaniak JD, Zheng SL. Discontinuation and non-publication of clinical trials in cardiovascular medicine. Int J Cardiol. 2017;244:309–315. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

