Tetrahydroindazole inhibitors of CDK2/cyclin complexes
- PMID: 33550184
- PMCID: PMC7954990
- DOI: 10.1016/j.ejmech.2021.113232
Tetrahydroindazole inhibitors of CDK2/cyclin complexes
Abstract
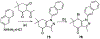
Over 50 tetrahydroindazoles were synthesized after 7-bromo-3,6,6-trimethyl-1-(pyridin-2-yl)-5,6,7,7a-tetrahydro-1H-indazol-4(3aH)-one (3) was identified as a hit compound in a high throughput screen for inhibition of CDK2 in complex with cyclin A. The activity of the most promising analogues was evaluated by inhibition of CDK2 enzyme complexes with various cyclins. Analogues 53 and 59 showed 3-fold better binding affinity for CDK2 and 2- to 10-fold improved inhibitory activity against CDK2/cyclin A1, E, and O compared to screening hit 3. The data from the enzyme and binding assays indicate that the binding of the analogues to a CDK2/cyclin complex is favored over binding to free CDK2. Computational analysis was used to predict a potential binding site at the CDK2/cyclin E1 interface.
Keywords: CDK2; Cyclins; Kinase inhibitors; Tetrahydroindazoles.
Copyright © 2021 Elsevier Masson SAS. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

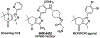
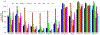


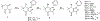


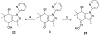

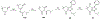
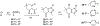


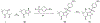
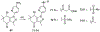
References
-
- Harper JW, Adams PD, Cyclin-dependent kinases, Chem. Rev, 101 (2001) 2511–2526. - PubMed
-
- Lim S, Kaldis P, Cdks, cyclins and CDKIs: Roles beyond cell cycle regulation, Development, 140 (2013) 3079–3093. - PubMed
-
- Weinberg RA, The retinoblastoma protein and cell cycle control, Cell, 81 (1995) 323–330. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

