Evidence and manipulation of O-GlcNAcylation in granulosa cells of bovine antral follicles†
- PMID: 33550377
- PMCID: PMC8023420
- DOI: 10.1093/biolre/ioab013
Evidence and manipulation of O-GlcNAcylation in granulosa cells of bovine antral follicles†
Abstract
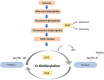
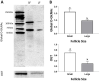
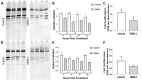
Glucose is a preferred energy substrate for metabolism by bovine granulosa cells (GCs). O-linked N-acetylglucosaminylation (O-GlcNAcylation), is a product of glucose metabolism that occurs as the hexosamine biosynthesis pathway (HBP) shunts O-GlcNAc sugars to serine and threonine residues of proteins. O-GlcNAcylation through the HBP is considered a nutrient sensing mechanism that regulates many cellular processes. Yet little is known of its importance in GCs. Here, O-GlcNAcylation in GCs and its effects on GC proliferation were determined. Bovine ovaries from a slaughterhouse, staged to the mid-to-late estrous period were used. Follicular fluid and GCs were aspirated from small (3-5 mm) and large (>10 mm) antral follicles. Freshly isolated GCs of small follicles exhibited greater expression of O-GlcNAcylation and O-GlcNAc transferase (OGT) than large follicles. Less glucose and more lactate was detectable in the follicular fluid of small versus large follicles. Culture of GCs revealed that inhibition of the HBP via the glutamine fructose-6-phosphate aminotransferase inhibitor, DON (50 μM), impaired O-GlcNAcylation and GC proliferation, regardless of follicle size. Direct inhibition of O-GlcNAcylation via the OGT inhibitor, OSMI-1 (50 μM), also prevented proliferation, but only in GCs of small follicles. Augmentation of O-GlcNAcylation via the O-GlcNAcase inhibitor, Thiamet-G (2.5 μM), had no effect on GC proliferation, regardless of follicle size. The results indicate GCs of bovine antral follicles undergo O-GlcNAcylation, and O-GlcNAcylation is associated with alterations of glucose and lactate in follicular fluid. Disruption of O-GlcNAcylation impairs GC proliferation. Thus, the HBP via O-GlcNAcylation constitutes a plausible nutrient-sensing pathway influencing bovine GC function and follicular growth.
Keywords: O-GlcNAcylation; bovine; glucose; granulosa cell; metabolism; ovary.
© The Author(s) 2021. Published by Oxford University Press on behalf of Society for the Study of Reproduction.
Figures




References
-
- Comer FI, Hart GW. O-glycosylation of nuclear and cytosolic proteins. Dynamic interplay between O-GlcNAc and O-phosphate. J Biol Chem 2000; 275:29179–29182. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

