Comparing the diagnostic performance of radiotracers in recurrent prostate cancer: a systematic review and network meta-analysis
- PMID: 33550425
- PMCID: PMC8263438
- DOI: 10.1007/s00259-021-05210-9
Comparing the diagnostic performance of radiotracers in recurrent prostate cancer: a systematic review and network meta-analysis
Erratum in
-
Correction to: Comparing the diagnostic performance of radiotracers in recurrent prostate cancer: a systematic review and network meta-analysis.Eur J Nucl Med Mol Imaging. 2021 Aug;48(9):3014-3016. doi: 10.1007/s00259-021-05300-8. Eur J Nucl Med Mol Imaging. 2021. PMID: 33748876 Free PMC article. No abstract available.
Abstract
Purpose: Many radiotracers are currently available for the detection of recurrent prostate cancer (rPC), yet many have not been compared head-to-head in comparative imaging studies. There is therefore an unmet need for evidence synthesis to guide evidence-based decisions in the selection of radiotracers. The objective of this study was therefore to assess the detection rate of various radiotracers for the rPC.
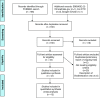
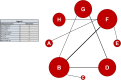
Methods: The PUBMED, EMBASE, and the EU and NIH trials databases were searched without date or language restriction for comparative imaging tracers for 13 radiotracers of principal interest. Key search terms included 18F-PSMA-1007, 18F-DCPFyl, 68Ga-PSMA-11, 18F-PSMA-11, 68Ga-PSMA-I&T, 68Ga-THP-PSMA, 64Cu-PSMA-617, 18F-JK-PSMA-7, 18F-Fluciclovine, 18F-FABC, 18F-Choline, 11C-Choline, and 68Ga-RM2. Studies reporting comparative imaging data in humans in rPC were selected. Single armed studies and matched pair analyses were excluded. Twelve studies with eight radiotracers were eligible for inclusion. Two independent reviewers screened all studies (using the PRISMA-NMA statement) for inclusion criteria, extracted data, and assessed risk of bias (using the QUADAS-2 tool). A network meta-analysis was performed using Markov-Chain Monte Carlo Bayesian analysis to obtain estimated detection rate odds ratios for each tracer combination.
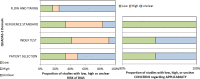
Results: A majority of studies were judged to be at risk of publication bias. With the exception of 18F-PSMA-1007, little difference in terms of detection rate was revealed between the three most commonly used PSMA-radiotracers (68Ga-PSMA-11, 18F-PSMA-1007, 18F-DCFPyl), which in turn showed clear superiority to choline and fluciclovine using the derived network.
Conclusion: Differences in patient-level detection rates were observed between PSMA- and choline-radiotracers. However, there is currently insufficient evidence to favour one of the four routinely used PSMA-radioligands (PSMA-11, PSMA-1007, PSMA-I&T, and DCFPyl) over another owing to the limited evidence base and risk of publication bias revealed by our systematic review. A further limitation was lack of reporting on diagnostic accuracy, which might favour radiotracers with low specificity in an analysis restricted only to detection rate. The NMA derived can be used to inform the design of future clinical trials and highlight areas where current evidence is weak.
Keywords: Choline; Comparative imaging; Network meta-analysis; PET/CT; PSMA; Positron emission tomography; Radiotracers.
Conflict of interest statement
The authors declare no competing interest.
Figures


Comment in
-
PSMA-PET: is the time to say goodbye to metabolic radiopharmaceuticals in prostate cancer?Eur J Nucl Med Mol Imaging. 2021 Jun;48(6):1709-1711. doi: 10.1007/s00259-021-05295-2. Epub 2021 Mar 13. Eur J Nucl Med Mol Imaging. 2021. PMID: 33715032 No abstract available.
-
Authors' reply: PSMA-PET: is the time to say goodbye to metabolic radiopharmaceuticals in prostate cancer?Eur J Nucl Med Mol Imaging. 2021 Jul;48(8):2307-2308. doi: 10.1007/s00259-021-05376-2. Epub 2021 May 4. Eur J Nucl Med Mol Imaging. 2021. PMID: 33948676 No abstract available.
References
-
- Hara T, Kosaka N, Kishi H. PET imaging of prostate cancer using Carbon-11-choline. J Nucl Med. 1998;39:990–995. - PubMed
-
- Calais J, Ceci F, Eiber M, Hope TA, Hofman MS, Rischpler C, et al. 18F-fluciclovine PET-CT and 68Ga-PSMA-11 PET-CT in patients with early biochemical recurrence after prostatectomy: a prospective, single-centre, single-arm, comparative imaging trial. The Lancet Oncology. 2019;20:1286–1294. doi: 10.1016/S1470-2045(19)30415-2. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

