Consensus of the Spanish society of laboratory medicine and the Spanish society of medical oncology on the methodology and criteria for evaluation of circulating tumour markers in breast cancer
- PMID: 33550504
- PMCID: PMC8192375
- DOI: 10.1007/s12094-020-02529-x
Consensus of the Spanish society of laboratory medicine and the Spanish society of medical oncology on the methodology and criteria for evaluation of circulating tumour markers in breast cancer
Abstract
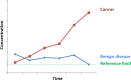
The measurement of circulating tumour markers (TMs) for the diagnosis or monitoring of breast cancer has sometimes been considered of limited utility. In addition to the overinterpretation of irrelevant changes in marker levels, the characteristics of the patient, the disease or other pathologies that can modify them are often not considered in their evaluation. On the other hand, there are recent data on the relationship of TMs with molecular subtypes and on their prognostic value, the knowledge of which may improve their clinical utility. This consensus article arises from a collaboration between the Spanish Society of Laboratory Medicine (SEQCML) and the Spanish Society of Medical Oncology (SEOM). It aims to improve the use and interpretation of circulating TMs in breast cancer. The text summarizes the current knowledge and available evidence on the subject and proposes a series of recommendations mainly focussed on the indication, the frequency of testing and the factors that should be considered for correctly interpreting changes in the levels of TMs.
Keywords: Biomarkers; Breast neoplasms; Carcinoembryonic antigen; Tumor suppressor; p53.
Conflict of interest statement
The authors declare that they did not have any conflict of interest related with this project.
Figures
Similar articles
-
[Consensus statement on biomarkers in breast cancer by the Spanish Society of Pathology and the Spanish Society of Medical Oncology].Rev Esp Patol. 2018 Apr-Jun;51(2):97-109. doi: 10.1016/j.patol.2017.12.002. Epub 2018 Mar 14. Rev Esp Patol. 2018. PMID: 29602380 Spanish.
-
Clinical practice guidelines for the use of tumor markers in breast and colorectal cancer. Adopted on May 17, 1996 by the American Society of Clinical Oncology.J Clin Oncol. 1996 Oct;14(10):2843-77. doi: 10.1200/JCO.1996.14.10.2843. J Clin Oncol. 1996. PMID: 8874347
-
Expert consensus of the Spanish Society of Pathology and the Spanish Society of Medical Oncology on the determination of biomarkers in pancreatic and biliary tract cancer.Clin Transl Oncol. 2022 Nov;24(11):2107-2119. doi: 10.1007/s12094-022-02873-0. Epub 2022 Aug 25. Clin Transl Oncol. 2022. PMID: 36008616 Free PMC article.
-
Serum cytokeratin fragment 21.1 (CYFRA 21.1) as tumour marker for breast cancer: comparison with carbohydrate antigen 15.3 (CA 15.3) and carcinoembryonic antigen (CEA).Clin Chem Lab Med. 2002 Mar;40(3):298-303. doi: 10.1515/CCLM.2002.047. Clin Chem Lab Med. 2002. PMID: 12005221
-
Biomarkers in breast cancer: A consensus statement by the Spanish Society of Medical Oncology and the Spanish Society of Pathology.Clin Transl Oncol. 2018 Jul;20(7):815-826. doi: 10.1007/s12094-017-1800-5. Epub 2017 Dec 22. Clin Transl Oncol. 2018. PMID: 29273958 Free PMC article. Review.
Cited by
-
Efficacy of a Supervised Exercise Program on Pain, Physical Function, and Quality of Life in Patients With Breast Cancer: Protocol for a Randomized Clinical Trial.JMIR Res Protoc. 2025 Mar 12;14:e63891. doi: 10.2196/63891. JMIR Res Protoc. 2025. PMID: 40073395 Free PMC article.
-
Technical Evaluation of the COBAS EGFR Semiquantitative Index (SQI) for Plasma cfDNA Testing in NSCLC Patients with EGFR Exon 19 Deletions.Diagnostics (Basel). 2021 Jul 22;11(8):1319. doi: 10.3390/diagnostics11081319. Diagnostics (Basel). 2021. PMID: 34441254 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous