Review of the immune mechanisms of preeclampsia and the potential of immune modulating therapy
- PMID: 33551128
- PMCID: PMC8062309
- DOI: 10.1016/j.humimm.2021.01.004
Review of the immune mechanisms of preeclampsia and the potential of immune modulating therapy
Abstract
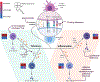
Successful pregnancy relies on maternal immunologic tolerance mechanisms limit maladaptive immune responses against the semi-allogeneic fetus and placenta and support fetal growth. Preeclampsia is a common disorder of pregnancy that affects 4-10% of pregnancies and is a leading cause of maternal and neonatal morbidity and mortality. Preeclampsia clinically manifests as maternal hypertension, proteinuria, and progressive multi-organ injury likely triggered by hypoxic injury to the placenta, resulting in local and systemic anti-angiogenic and inflammatory factor production. Despite the steady rising rates of preeclampsia in the United States, effective treatment options are limited to delivery, which improves maternal status often at the cost of prematurity in the newborn. Preeclampsia also increases the lifelong risk of cardiovascular disease for both mother and infant. Thus, identifying new therapeutic targets is a high priority area to improve maternal, fetal, and infant health outcomes. Immune abnormalities in the placenta and in the maternal circulation have been reported to precede the clinical onset of disease. In particular, excessive systemic and placental complement activation and impaired adaptive T cell tolerance with Th1/Th2/Th17/Treg imbalance has been reported in humans and in animal models of preeclampsia. In this review, we focus on the evidence for the immune origins of preeclampsia, discuss the promise of immune modulating therapy for prevention or treatment, and highlight key areas for future research.
Keywords: Complement; Immune modulation; Preeclampsia; Pregnancy; Regulatory T cells.
Copyright © 2021 American Society for Histocompatibility and Immunogenetics. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest Dr. Karumanchi is co-listed as co-inventors on patents related to preeclampsia biomarkers that are held at Beth Israel Deaconess Medical Center. He has financial interest in Aggamin LLC and also reports serving as a consultant to Roche Diagnostics and Thermofisher. Dr. Karumanchi has received research funding from Siemens and Thermofisher. Other authors report no conflicts.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

