Epitokous metamorphosis, reproductive swimming, and early development of the estuarine polychaete, Neanthes glandicincta Southern, 1921 (Annelida, Nereididae) on the east coast of the Malay Peninsula
- PMID: 33551646
- PMCID: PMC7835201
- DOI: 10.3897/zookeys.1011.59780
Epitokous metamorphosis, reproductive swimming, and early development of the estuarine polychaete, Neanthes glandicincta Southern, 1921 (Annelida, Nereididae) on the east coast of the Malay Peninsula
Abstract
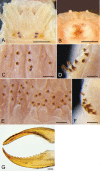
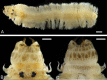
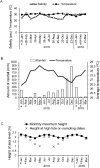
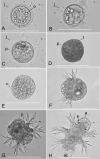
The reproductive and developmental characteristics of the nereidid polychaete, Neanthes glandicincta Southern, 1921, commonly recorded in tropical estuaries in the Indo-West Pacific, were examined from Malaysia (the mangrove area of Kuala Ibai, Terengganu) and Thailand (the Lower Songkhla Lagoon) on the east coast of the Malay Peninsula. Epitokous metamorphosis of fully mature males and females and their reproductive swimming behaviour were recorded based on ten Malaysian epitokous specimens, which were collected at night during spring tides in a period of January 2018 to March 2019. Six Thailand epitokes were obtained in February and March 2006 by the laboratory rearing of immature worms. Epitokous metamorphosis is characterised by the enlargement of eyes in both sexes, division of the body into three parts and modification of parapodia with additional lobes in the mid-body of males, and replacement of atokous chaetae in the mid-body by epitokous natatory chaetae, completely in males and incompletely in females. The diameter of coelomic unfertilised eggs in females was 100-140 µm. After fertilisation, each egg formed a jelly layer, inside which embryonic development progressed. Trochophores hatched out of the jelly layer, entering a short free-swimming larval phase followed by demersal life at the early stage of 3-chaetiger nectochaeta one day after fertilisation. Then, the larvae entered benthic life as juveniles, crawling on the bottom, at the late stage of 3-chaetiger nectochaeta two days after fertilisation. The results indicate that N. glandicincta has an annual life cycle, which is usually completed within an estuary with limited larval dispersal ability.
Keywords: Kuala Ibai; Songkhla Lagoon; South China Sea; natatory chaetae; pelagic larvae; trochophore.
Siti Syazwani Azmi, Yusof Shuaib Ibrahim, Saowapa Angsupanich, Pornsan Sumpuntarat, Masanori Sato.
Figures





References
-
- Akhir MF, Sinha PC, Hussain ML. (2011) Seasonal variation of South China Sea physical characteristics of the east coast of Peninsular Malaysia from 2002–2010 datasets. International Journal of Environmental Sciences 2(2): 569–575.
-
- Angsupanich S, Rakkheaw S. (1997) Seasonal variation of phytoplankton community in Thale Sap Songkhla, a lagoonal lake in southern Thailand. Netherlands Journal of Aquatic Ecology 30: 297–307. 10.1007/BF02085873 - DOI
-
- Chan WMF. (2009) New nereidid records (Annelida: Polychaeta) from mangroves and sediment flats of Singapore. Raffles Bulletin of Zoology, Supplement 22: 159–172.
-
- Fauvel P. (1932) AnnelidaPolychaeta of the Indian Museum. Memoirs of the Indian Museum, Calcutta 12(1): 1–262.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
