Top-Down Inference in the Auditory System: Potential Roles for Corticofugal Projections
- PMID: 33551756
- PMCID: PMC7862336
- DOI: 10.3389/fncir.2020.615259
Top-Down Inference in the Auditory System: Potential Roles for Corticofugal Projections
Abstract
It has become widely accepted that humans use contextual information to infer the meaning of ambiguous acoustic signals. In speech, for example, high-level semantic, syntactic, or lexical information shape our understanding of a phoneme buried in noise. Most current theories to explain this phenomenon rely on hierarchical predictive coding models involving a set of Bayesian priors emanating from high-level brain regions (e.g., prefrontal cortex) that are used to influence processing at lower-levels of the cortical sensory hierarchy (e.g., auditory cortex). As such, virtually all proposed models to explain top-down facilitation are focused on intracortical connections, and consequently, subcortical nuclei have scarcely been discussed in this context. However, subcortical auditory nuclei receive massive, heterogeneous, and cascading descending projections at every level of the sensory hierarchy, and activation of these systems has been shown to improve speech recognition. It is not yet clear whether or how top-down modulation to resolve ambiguous sounds calls upon these corticofugal projections. Here, we review the literature on top-down modulation in the auditory system, primarily focused on humans and cortical imaging/recording methods, and attempt to relate these findings to a growing animal literature, which has primarily been focused on corticofugal projections. We argue that corticofugal pathways contain the requisite circuitry to implement predictive coding mechanisms to facilitate perception of complex sounds and that top-down modulation at early (i.e., subcortical) stages of processing complement modulation at later (i.e., cortical) stages of processing. Finally, we suggest experimental approaches for future studies on this topic.
Keywords: auditory; colliculus; cortex; descending; medial geniculate body; speech perception; thalamus; top-down.
Copyright © 2021 Asilador and Llano.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
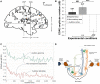
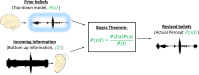

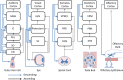

References
-
- Adams R. A., Brown H. R., Friston K. J. (2014). Bayesian inference, predictive coding and delusions. Avant 5, 51–88. 10.26913/50302014.0112.0004 - DOI
-
- Amato G., La V. G., Enia F. (1969). The control exerted by the auditory cortex on the activity of the medial geniculate body and inferior colliculus. Arch. Sci. Biol. 53, 291–313. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

