The "C3aR Antagonist" SB290157 is a Partial C5aR2 Agonist
- PMID: 33551801
- PMCID: PMC7859635
- DOI: 10.3389/fphar.2020.591398
The "C3aR Antagonist" SB290157 is a Partial C5aR2 Agonist
Abstract
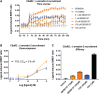
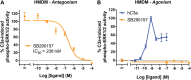
Innate immune complement activation generates the C3 and C5 protein cleavage products C3a and C5a, defined classically as anaphylatoxins. C3a activates C3aR, while C5a activates two receptors (C5aR1 and C5aR2) to exert their immunomodulatory activities. The non-peptide compound, SB290157, was originally reported in 2001 as the first C3aR antagonist. In 2005, the first report on the non-selective nature of SB290157 was published, where the compound exerted clear agonistic, not antagonistic, activity in variety of cells. Other studies also documented the non-selective activities of this drug in vivo. These findings severely hamper data interpretation regarding C3aR when using this compound. Unfortunately, given the dearth of C3aR inhibitors, SB290157 still remains widely used to explore C3aR biology (>70 publications to date). Given these issues, in the present study we aimed to further explore SB290157's pharmacological selectivity by screening the drug against three human anaphylatoxin receptors, C3aR, C5aR1 and C5aR2, using cell models. We identified that SB290157 exerts partial agonist activity at C5aR2 by mediating β-arrestin recruitment at higher compound doses. This translated to a functional outcome in both human and mouse primary macrophages, where SB290157 significantly dampened C5a-induced ERK signaling. We also confirmed that SB290157 acts as a potent agonist at human C3aR in transfected cells, but as an antagonist in primary human macrophages. Our results therefore provide even more caution against using SB290157 as a research tool to explore C3aR function. Given the reported immunomodulatory and anti-inflammatory activities of C5aR2 agonism, any function observed with SB290157 could be due to these off-target activities.
Keywords: C3a anaphylatoxin; C3aR; C5a anaphylatoxin; C5aR1; C5aR2; SB290157; complement; complement C3a.
Copyright © 2021 Li, Kumar, Clark, Lee and Woodruff.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Ames R. S., Lee D., Foley J. J., Jurewicz A. J., Tornetta M. A., Bautsch W., et al. (2001). Identification of a selective nonpeptide antagonist of the anaphylatoxin C3a receptor that demonstrates antiinflammatory activity in animal models. J. Immunol. 166 (10), 6341 10.4049/jimmunol.166.10.6341 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

