A Putative Serine Protease is Required to Initiate the RIPK3-MLKL-Mediated Necroptotic Death Pathway in Neutrophils
- PMID: 33551816
- PMCID: PMC7860068
- DOI: 10.3389/fphar.2020.614928
A Putative Serine Protease is Required to Initiate the RIPK3-MLKL-Mediated Necroptotic Death Pathway in Neutrophils
Abstract
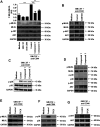
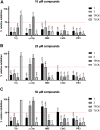
Adhesion receptors, such as CD44, have been shown to activate receptor interacting protein kinase-3 (RIPK3)-mixed lineage kinase-like (MLKL) signaling, leading to a non-apoptotic cell death in human granulocyte/macrophage colony-stimulating factor (GM-CSF) - primed neutrophils. The signaling events of this necroptotic pathway, however, remain to be investigated. In the present study, we report the design, synthesis, and characterization of a series of novel serine protease inhibitors. Two of these inhibitors, compounds 1 and 3, were able to block CD44-triggered necroptosis in GM-CSF-primed neutrophils. Both inhibitors prevented the activation of MLKL, p38 mitogen-activated protein kinase (MAPK) and phosphatidylinositol 3'-kinase (PI3K), hence blocking the increased levels of reactive oxygen species (ROS) required for cell death. Although compounds one and three partially inhibited isolated human neutrophil elastase (HNE) activity, we obtained no pharmacological evidence that HNE is involved in the initiation of this death pathway within a cellular context. Interestingly, neither serine protease inhibitor had any effect on FAS receptor-mediated apoptosis. Taken together, these results suggest that a serine protease is involved in non-apoptotic CD44-triggered RIPK3-MLKL-dependent neutrophil cell death, but not FAS receptor-mediated caspase-dependent apoptosis. Thus, a pharmacological block on serine proteases might be beneficial for preventing exacerbation of disease in neutrophilic inflammatory responses.
Keywords: necroptosis; neutrophil; serine protease; signal transduction; small molecule inhibitor.
Copyright © 2021 Simon, Wang, Avsec, Obreza, Yousefi and Mlinaric-Rascan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

