Natural Compounds in Sex Hormone-Dependent Cancers: The Role of Triterpenes as Therapeutic Agents
- PMID: 33552000
- PMCID: PMC7859451
- DOI: 10.3389/fendo.2020.612396
Natural Compounds in Sex Hormone-Dependent Cancers: The Role of Triterpenes as Therapeutic Agents
Abstract
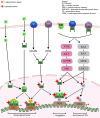
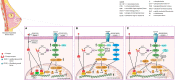
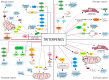
Sex hormone-dependent cancers currently contribute to the high number of cancer-related deaths worldwide. The study and elucidation of the molecular mechanisms underlying the progression of these tumors was a double-edged sword, leading to the expansion and development of new treatment options, with the cost of triggering more aggressive, therapy resistant relapses. The interaction of androgen, estrogen and progesterone hormones with specific receptors (AR, ER, PR) has emerged as a key player in the development and progression of breast, ovarian, prostate and endometrium cancers. Sex hormone-dependent cancers share a common and rather unique carcinogenesis mechanism involving the active role of endogenous and exogenous sex hormones to maintain high mitotic rates and increased cell proliferation thus increasing the probability of aberrant gene occurrence and accumulation highly correlated with abnormal cell division and the occurrence of malignant phenotypes. Cancer related hormone therapy has evolved, currently being associated with the blockade of other signaling pathways often associated with carcinogenesis and tumor progression in cancers, with promising results. However, despite the established developments, there are still several shortcomings to be addressed. Triterpenes are natural occurring secondary metabolites biosynthesized by various pathways starting from squalene cyclization. Due to their versatile therapeutic potential, including the extensively researched antiproliferative effect, these compounds are most definitely a cornerstone in the research and development of new natural/semisynthetic anticancer therapies. The present work thoroughly describes the ongoing research related to the antitumor activity of triterpenes in sex hormone-dependent cancers. Also, the current review highlights both the biological activity of various triterpenoid compounds and their featured mechanisms of action correlated with important chemical structural features.
Keywords: antiproliferative activity; hormone-dependent cancers; phytocompounds; therapeutic agents; triterpenes.
Copyright © 2021 Şoica, Voicu, Ghiulai, Dehelean, Racoviceanu, Trandafirescu, Roșca, Nistor, Mioc and Mioc.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Méndez-Cuesta C, Campos A, Sánchez D, Pérez-González C, Gutiérrez S. Cytotoxic and Antitumoral Activities of Compounds Isolated from Cucurbitaceae Plants. In: Pharmacognosy - Medicinal Plants. IntechOpen; (2018). 10.5772/intechopen.82213 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

