Functional Insights From KpfR, a New Transcriptional Regulator of Fimbrial Expression That Is Crucial for Klebsiella pneumoniae Pathogenicity
- PMID: 33552015
- PMCID: PMC7861041
- DOI: 10.3389/fmicb.2020.601921
Functional Insights From KpfR, a New Transcriptional Regulator of Fimbrial Expression That Is Crucial for Klebsiella pneumoniae Pathogenicity
Abstract
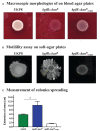
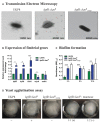
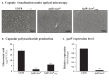
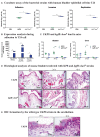
Although originally known as an opportunistic pathogen, Klebsiella pneumoniae has been considered a worldwide health threat nowadays due to the emergence of hypervirulent and antibiotic-resistant strains capable of causing severe infections not only on immunocompromised patients but also on healthy individuals. Fimbriae is an essential virulence factor for K. pneumoniae, especially in urinary tract infections (UTIs), because it allows the pathogen to adhere and invade urothelial cells and to form biofilms on biotic and abiotic surfaces. The importance of fimbriae for K. pneumoniae pathogenicity is highlighted by the large number of fimbrial gene clusters on the bacterium genome, which requires a coordinated and finely adjusted system to control the synthesis of these structures. In this work, we describe KpfR as a new transcriptional repressor of fimbrial expression in K. pneumoniae and discuss its role in the bacterium pathogenicity. K. pneumoniae with disrupted kpfR gene exhibited a hyperfimbriated phenotype with enhanced biofilm formation and greater adhesion to and replication within epithelial host cells. Nonetheless, the mutant strain was attenuated for colonization of the bladder in a murine model of urinary tract infection. These results indicate that KpfR is an important transcriptional repressor that, by negatively controlling the expression of fimbriae, prevents K. pneumoniae from having a hyperfimbriated phenotype and from being recognized and eliminated by the host immune system.
Keywords: Klebsiella pneumonia; adherence; biofilms; coculture; fimbriae; host-microbe interactions; transcriptional regulation; urinary tract infection.
Copyright © 2021 Gomes, Pacheco, Santos, Pereira, Ribeiro, Darrieux and Ferraz.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Conserved FimK Truncation Coincides with Increased Expression of Type 3 Fimbriae and Cultured Bladder Epithelial Cell Association in Klebsiella quasipneumoniae.J Bacteriol. 2022 Sep 20;204(9):e0017222. doi: 10.1128/jb.00172-22. Epub 2022 Aug 25. J Bacteriol. 2022. PMID: 36005809 Free PMC article.
-
SdiA, a Quorum-Sensing Regulator, Suppresses Fimbriae Expression, Biofilm Formation, and Quorum-Sensing Signaling Molecules Production in Klebsiella pneumoniae.Front Microbiol. 2021 Jun 21;12:597735. doi: 10.3389/fmicb.2021.597735. eCollection 2021. Front Microbiol. 2021. PMID: 34234747 Free PMC article.
-
Identification of a conserved chromosomal region encoding Klebsiella pneumoniae type 1 and type 3 fimbriae and assessment of the role of fimbriae in pathogenicity.Infect Immun. 2009 Nov;77(11):5016-24. doi: 10.1128/IAI.00585-09. Epub 2009 Aug 24. Infect Immun. 2009. PMID: 19703972 Free PMC article.
-
Klebsiella pneumoniae and type 3 fimbriae: nosocomial infection, regulation and biofilm formation.Future Microbiol. 2012 Aug;7(8):991-1002. doi: 10.2217/fmb.12.74. Future Microbiol. 2012. PMID: 22913357 Review.
-
Klebsiella pneumoniae: Going on the Offense with a Strong Defense.Microbiol Mol Biol Rev. 2016 Jun 15;80(3):629-61. doi: 10.1128/MMBR.00078-15. Print 2016 Sep. Microbiol Mol Biol Rev. 2016. PMID: 27307579 Free PMC article. Review.
Cited by
-
Four Types of ST11 Novel Mutations From Increasing Carbapenem-Resistant Klebsiella pneumoniae in Guangdong, 2016-2020.Front Microbiol. 2021 Oct 1;12:702941. doi: 10.3389/fmicb.2021.702941. eCollection 2021. Front Microbiol. 2021. PMID: 34659140 Free PMC article.
-
Medical Device-Associated Biofilm Infections and Multidrug-Resistant Pathogens.Pathogens. 2024 May 8;13(5):393. doi: 10.3390/pathogens13050393. Pathogens. 2024. PMID: 38787246 Free PMC article. Review.
-
Current Stage in the Development of Klebsiella pneumoniae Vaccines.Infect Dis Ther. 2021 Dec;10(4):2157-2175. doi: 10.1007/s40121-021-00533-4. Epub 2021 Sep 2. Infect Dis Ther. 2021. PMID: 34476772 Free PMC article. Review.
-
Regulation of biofilm formation in Klebsiella pneumoniae.Front Microbiol. 2023 Sep 7;14:1238482. doi: 10.3389/fmicb.2023.1238482. eCollection 2023. Front Microbiol. 2023. PMID: 37744914 Free PMC article. Review.
-
A unique combination of natural fatty acids from Hermetia illucens fly larvae fat effectively combats virulence factors and biofilms of MDR hypervirulent mucoviscus Klebsiella pneumoniae strains by increasing Lewis acid-base/van der Waals interactions in bacterial wall membranes.Front Cell Infect Microbiol. 2024 Jul 25;14:1408179. doi: 10.3389/fcimb.2024.1408179. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39119288 Free PMC article.
References
-
- Ares M. A., Fernández-Vázquez J. L., Rosales-Reyes R., Jarillo-Quijada M. D., von Bargen K., Torres J., et al. (2016). H-NS nucleoid protein controls virulence features of Klebsiella pneumoniae by regulating the expression of type 3 pili and the capsule polysaccharide. Front. Cell. Infect. Microbiol. 6:13. 10.3389/fcimb.2016.00013 - DOI - PMC - PubMed
-
- Balestrino D., Ghigo J.-M., Charbonnel N., Haagensen J. A. J., Forestier C. (2008). The characterization of functions involved in the establishment and maturation of Klebsiella pneumoniae in vitro biofilm reveals dual roles for surface exopolysaccharides. Environ. Microbiol. 10 685–701. 10.1111/j.1462-2920.2007.01491.x - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

