Co-dependent and Interdigitated: Dual Quorum Sensing Systems Regulate Conjugative Transfer of the Ti Plasmid and the At Megaplasmid in Agrobacterium tumefaciens 15955
- PMID: 33552018
- PMCID: PMC7856919
- DOI: 10.3389/fmicb.2020.605896
Co-dependent and Interdigitated: Dual Quorum Sensing Systems Regulate Conjugative Transfer of the Ti Plasmid and the At Megaplasmid in Agrobacterium tumefaciens 15955
Abstract
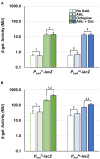
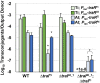
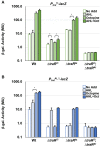
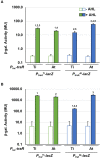
Members of the Rhizobiaceae, often carry multiple secondary replicons in addition to the primary chromosome with compatible repABC-based replication systems. Unlike secondary chromosomes and chromids, repABC-based megaplasmids and plasmids can undergo copy number fluctuations and are capable of conjugative transfer in response to environmental signals. Several Agrobacterium tumefaciens lineages harbor three secondary repABC-based replicons, including a secondary chromosome (often linear), the Ti (tumor-inducing) plasmid and the At megaplasmid. The Ti plasmid is required for virulence and encodes a conjugative transfer (tra) system that is strictly regulated by a subset of plant-tumor released opines and a well-described acyl-homoserine lactone (AHL)-based quorum-sensing mechanism. The At plasmids are generally not required for virulence, but carry genes that enhance rhizosphere survival, and these plasmids are often conjugatively proficient. We report that the At megaplasmid of the octopine-type strain A. tumefaciens 15955 encodes a quorum-controlled conjugation system that directly interacts with the paralogous quorum sensing system on the co-resident Ti plasmid. Both the pAt15955 and pTi15955 plasmids carry homologs of a TraI-type AHL synthase, a TraR-type AHL-responsive transcription activator, and a TraM-type anti-activator. The traI genes from both pTi15955 and pAt15955 can direct production of the inducing AHL (3-octanoyl-L-homoserine lactone) and together contribute to the overall AHL pool. The TraR protein encoded on each plasmid activates AHL-responsive transcription of target tra gene promoters. The pAt15955 TraR can cross-activate tra genes on the Ti plasmid as strongly as its cognate tra genes, whereas the pTi15955 TraR is preferentially biased toward its own tra genes. Putative tra box elements are located upstream of target promoters, and comparing between plasmids, they are in similar locations and share an inverted repeat structure, but have distinct consensus sequences. The two AHL quorum sensing systems have a combinatorial effect on conjugative transfer of both plasmids. Overall, the interactions described here have implications for the horizontal transfer and evolutionary stability of both plasmids and, in a broad sense, are consistent with other repABC systems that often have multiple quorum-sensing controlled secondary replicons.
Keywords: conjugative transfer; gene regulation; plasmids; quorum sensing (QS); repABC system.
Copyright © 2021 Barton, Eagan, Nieves-Otero, Reynolds, Platt and Fuqua.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Alt-Mörbe J., Stryker J. L., Fuqua C., Li P.-L., Farrand S. K., Winans S. C. (1996). The conjugal transfer system of Agrobacterium tumefaciens octopine-type Ti plasmids is closely related to the transfer system of an IncP plasmid and distantly related to Ti plasmid vir genes. J. Bacteriol. 178, 4248–4257. 10.1128/JB.178.14.4248-4257.1996 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

