Localization and Composition of Fructans in Stem and Rhizome of Agave tequilana Weber var. azul
- PMID: 33552101
- PMCID: PMC7855178
- DOI: 10.3389/fpls.2020.608850
Localization and Composition of Fructans in Stem and Rhizome of Agave tequilana Weber var. azul
Abstract
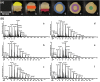
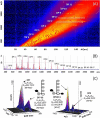
Methodology combining mass spectrometry imaging (MSI) with ion mobility separation (IMS) has emerged as a biological imaging technique due to its versatility, sensitivity and label-free approach. This technique has been shown to separate isomeric compounds such as lipids, amino acids, carboxylic acids and carbohydrates. This report describes mass spectrometry imaging in combination with traveling-wave ion mobility separation and matrix-assisted laser desorption/ionization (MALDI). Positive ionization mode was used to locate fructans on tissue printed sections of Agave rhizome and stem tissue and distinguished fructan isoforms. Here we show the location of fructans ranging from DP3 to DP17 to be differentially abundant across the stem tissue and for the first time, experimental collision cross sections of endogenous fructan structures have been collected, revealing at least two isoforms for fructans of DP4, DP5, DP6, DP7, DP8, DP10, and DP11. This demonstrates that complex fructans such as agavins can be located and their isoforms resolved using a combination of MALDI, IMS, and MSI, without the need for extraction or derivatization. Use of this methodology uncovered patterns of fructan localization consistent with functional differences where higher DP fructans are found toward the central section of the stem supporting a role in long term carbohydrate storage whereas lower DP fructans are concentrated in the highly vascularized central core of rhizomes supporting a role in mobilization of carbohydrates from the mother plant to developing offsets. Tissue specific patterns of expression of genes encoding enzymes involved in fructan metabolism are consistent with fructan structures and localization.
Keywords: Agave tequilana; collision cross section; degree of polymerization; fructan; fructan isoform; fructan metabolism; ion mobility separation; mass spectrometry imaging.
Copyright © 2021 Pérez-López, Simpson, Clench, Gomez-Vargas and Ordaz-Ortiz.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Acosta-Domínguez L., Alamilla-Beltrán L., Calderón-Domínguez G., Jiménez-Aparicio A. R., Gutiérrez-López G. F., Azuara-Nieto E. (2018). Determination Of Total And Incipient Solubilization Point Of Fructans Extracted Of A. tequilana Weber var. azul. Rev. Mexic. Ingen. Quím. 17 379–388. 10.24275/uam/izt/dcbi/revmexingquim/2018v17n1/acosta - DOI
-
- Antonio C., Larson T., Gilday A., Graham I., Bergström E., Thomas-Oates J. (2008). Hydrophilic interaction chromatography/electrospray mass spectrometry analysis of carbohydrate-related metabolites from Arabidopsis thaliana leaf tissue. Rap. Commun. Mass Spectr. RCM 22 1399–1407. 10.1002/rcm.3519 - DOI - PubMed
-
- Arrizon J., Morel S., Gschaedler A., Monsan P. (2010). Comparison of the water-soluble carbohydrate composition and fructan structures of Agave tequilana plants of different ages. Food Chem. 122 123–130. 10.1016/j.foodchem.2010.02.028 - DOI
-
- Bhatia I. S., Nandra K. S. (1979). Studies on fructosyl transferase from Agave americana. Phytochemistry 18 923–927. 10.1016/s0031-9422(00)91450-x - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

