l-Isoaspartyl Methyltransferase Deficiency in Zebrafish Leads to Impaired Calcium Signaling in the Brain
- PMID: 33552132
- PMCID: PMC7859441
- DOI: 10.3389/fgene.2020.612343
l-Isoaspartyl Methyltransferase Deficiency in Zebrafish Leads to Impaired Calcium Signaling in the Brain
Abstract
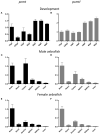
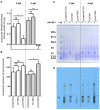
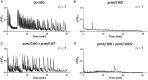
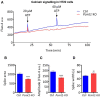
Isomerization of l-aspartyl and l-asparaginyl residues to l-isoaspartyl residues is one type of protein damage that can occur under physiological conditions and leads to conformational changes, loss of function, and enhanced protein degradation. Protein l-isoaspartyl methyltransferase (PCMT) is a repair enzyme whose action initiates the reconversion of abnormal l-isoaspartyl residues to normal l-aspartyl residues in proteins. Many lines of evidence support a crucial role for PCMT in the brain, but the mechanisms involved remain poorly understood. Here, we investigated PCMT activity and function in zebrafish, a vertebrate model that is particularly well-suited to analyze brain function using a variety of techniques. We characterized the expression products of the zebrafish PCMT homologous genes pcmt and pcmtl. Both zebrafish proteins showed a robust l-isoaspartyl methyltransferase activity and highest mRNA transcript levels were found in brain and testes. Zebrafish morphant larvae with a knockdown in both the pcmt and pcmtl genes showed pronounced morphological abnormalities, decreased survival, and increased isoaspartyl levels. Interestingly, we identified a profound perturbation of brain calcium homeostasis in these morphants. An abnormal calcium response upon ATP stimulation was also observed in mouse hippocampal HT22 cells knocked out for Pcmt1. This work shows that zebrafish is a promising model to unravel further facets of PCMT function and demonstrates, for the first time in vivo, that PCMT plays a pivotal role in the regulation of calcium fluxes.
Keywords: HT22 cells; calcium signaling; isoaspartyl; protein repair; zebrafish.
Copyright © 2021 Soliman, Cordero-Maldonado, Martins, Moein, Conrotte, Warmack, Skupin, Crawford, Clarke and Linster.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Structural organization and developmental expression of the protein isoaspartyl methyltransferase gene from Drosophila melanogaster.Insect Biochem Mol Biol. 1997 Jan;27(1):49-54. doi: 10.1016/s0965-1748(96)00071-9. Insect Biochem Mol Biol. 1997. PMID: 9061928
-
Protein isoaspartate methyltransferase prevents apoptosis induced by oxidative stress in endothelial cells: role of Bcl-Xl deamidation and methylation.PLoS One. 2008 Sep 22;3(9):e3258. doi: 10.1371/journal.pone.0003258. PLoS One. 2008. Retraction in: PLoS One. 2018 Nov 8;13(11):e0207530. doi: 10.1371/journal.pone.0207530. PMID: 18806875 Free PMC article. Retracted.
-
Methylation of atypical protein aspartyl residues during the stress response of HeLa cells.J Cell Physiol. 1992 Nov;153(2):297-304. doi: 10.1002/jcp.1041530209. J Cell Physiol. 1992. PMID: 1429850
-
PROTEIN l-ISOASPARTYL METHYLTRANSFERASE (PIMT) in plants: regulations and functions.Biochem J. 2020 Nov 27;477(22):4453-4471. doi: 10.1042/BCJ20200794. Biochem J. 2020. PMID: 33245750 Review.
-
Damaged proteins bearing L-isoaspartyl residues and aging: a dynamic equilibrium between generation of isomerized forms and repair by PIMT.Curr Aging Sci. 2011 Feb;4(1):8-18. Curr Aging Sci. 2011. PMID: 21204776 Review.
Cited by
-
Isoaspartate-containing galanin in rat hypothalamus.Commun Chem. 2025 Mar 8;8(1):72. doi: 10.1038/s42004-025-01475-5. Commun Chem. 2025. PMID: 40057589 Free PMC article.
-
Untargeted Discovery and Localization of Isomerized Residues in Neuropeptides.Anal Chem. 2025 Aug 19;97(32):17570-17579. doi: 10.1021/acs.analchem.5c02612. Epub 2025 Aug 7. Anal Chem. 2025. PMID: 40774382 Free PMC article.
-
PIMT-Mediated Labeling of l-Isoaspartic Acid with Tris Facilitates Identification of Isomerization Sites in Long-Lived Proteins.J Am Soc Mass Spectrom. 2022 Mar 2;33(3):548-556. doi: 10.1021/jasms.1c00355. Epub 2022 Feb 3. J Am Soc Mass Spectrom. 2022. PMID: 35113558 Free PMC article.
-
PCMT1 regulates the migration, invasion, and apoptosis of prostate cancer through modulating the PI3K/AKT/GSK-3β pathway.Aging (Albany NY). 2023 Oct 27;15(20):11654-11671. doi: 10.18632/aging.205152. Epub 2023 Oct 27. Aging (Albany NY). 2023. PMID: 37899170 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

