Pattern of circulating SARS-CoV-2-specific antibody-secreting and memory B-cell generation in patients with acute COVID-19
- PMID: 33552508
- PMCID: PMC7848539
- DOI: 10.1002/cti2.1245
Pattern of circulating SARS-CoV-2-specific antibody-secreting and memory B-cell generation in patients with acute COVID-19
Abstract
Objectives: To predict the spread of coronavirus disease (COVID-19), information regarding the immunological memory for disease-specific antigens is necessary. The possibility of reinfection, as well as the efficacy of vaccines for COVID-19 that are currently under development, will largely depend on the quality and longevity of immunological memory in patients. To elucidate the process of humoral immunity development, we analysed the generation of plasmablasts and virus receptor-binding domain (RBD)-specific memory B (Bmem) cells in patients during the acute phase of COVID-19.
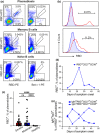
Methods: The frequencies of RBD-binding plasmablasts and RBD-specific antibody-secreting cells (ASCs) in the peripheral blood samples collected from patients with COVID-19 were measured using flow cytometry and the ELISpot assay.
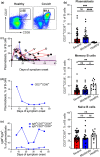
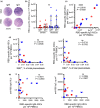
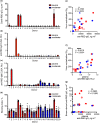
Results: The acute phase of COVID-19 was characterised by the transient appearance of total as well as RBD-binding plasmablasts. ELISpot analysis indicated that most patients exhibited a spontaneous secretion of RBD-specific ASCs in the circulation with good correlation between the IgG and IgM subsets. IL-21/CD40L stimulation of purified B cells induced the activation and proliferation of Bmem cells, which led to the generation of plasmablast phenotypic cells as well as RBD-specific ASCs. No correlation was observed between the frequency of Bmem cell-derived and spontaneous ASCs, suggesting that the two types of ASCs were weakly associated with each other.
Conclusion: Our findings reveal that SARS-CoV-2-specific Bmem cells are generated during the acute phase of COVID-19. These findings can serve as a basis for further studies on the longevity of SARS-CoV-2-specific B-cell memory.
Keywords: SARS‐CoV‐2; antibody‐secreting cell; memory B cell; plasmablast; virus‐neutralising antibodies.
© 2021 The Authors. Clinical & Translational Immunology published by John Wiley & Sons Australia, Ltd on behalf of Australian and New Zealand Society for Immunology, Inc.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Ju B, Zhang QI, Ge J et al Human neutralizing antibodies elicited by SARS‐CoV‐2 infection. Nature 2020; 584: 115–119. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
