Biomedically Relevant Applications of Bolaamphiphiles and Bolaamphiphile-Containing Materials
- PMID: 33553103
- PMCID: PMC7855593
- DOI: 10.3389/fchem.2020.604151
Biomedically Relevant Applications of Bolaamphiphiles and Bolaamphiphile-Containing Materials
Abstract
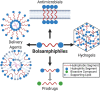
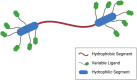
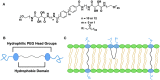
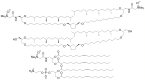
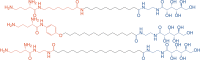
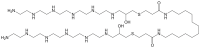
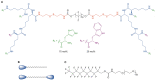
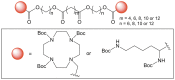
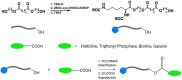
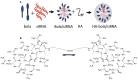
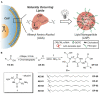
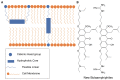
Bolaamphiphiles (BAs) are structurally segmented molecules with rich assembly characteristics and diverse physical properties. Interest in BAs as standalone active agents or as constituents of more complex therapeutic formulations has increased substantially in recent years. The preorganized amphiphilicity of BAs allows for a range of biological activities including applications that rely on multivalency. This review summarizes BA-related research in biomedically relevant areas. In particular, we review BA-related literature in four areas: gene delivery, antimicrobial materials, hydrogels, and prodrugs. We also discuss several distinguishing characteristics of BAs that impact their utility as biomedically relevant compounds.
Keywords: antimicrobials; biomaterials; bolaamphiphiles; bolaform amphiphile; gene delivery; prodrugs.
Copyright © 2021 Hughes, Miller, Wallace, Vemuri and Iovine.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

