Pulsatile Flow-Induced Fatigue-Resistant Photopolymerizable Hydrogels for the Treatment of Intracranial Aneurysms
- PMID: 33553124
- PMCID: PMC7855579
- DOI: 10.3389/fbioe.2020.619858
Pulsatile Flow-Induced Fatigue-Resistant Photopolymerizable Hydrogels for the Treatment of Intracranial Aneurysms
Abstract
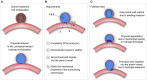
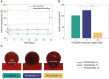
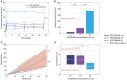
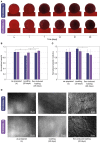
An alternative intracranial aneurysm embolic agent is emerging in the form of hydrogels due to their ability to be injected in liquid phase and solidify in situ. Hydrogels have the ability to fill an aneurysm sac more completely compared to solid implants such as those used in coil embolization. Recently, the feasibility to implement photopolymerizable poly(ethylene glycol) dimethacrylate (PEGDMA) hydrogels in vitro has been demonstrated for aneurysm application. Nonetheless, the physical and mechanical properties of such hydrogels require further characterization to evaluate their long-term integrity and stability to avoid implant compaction and aneurysm recurrence over time. To that end, molecular weight and polymer content of the hydrogels were tuned to match the elastic modulus and compliance of aneurysmal tissue while minimizing the swelling volume and pressure. The hydrogel precursor was injected and photopolymerized in an in vitro aneurysm model, designed by casting polydimethylsiloxane (PDMS) around 3D printed water-soluble sacrificial molds. The hydrogels were then exposed to a fatigue test under physiological pulsatile flow, inducing a combination of circumferential and shear stresses. The hydrogels withstood 5.5 million cycles and no significant weight loss of the implant was observed nor did the polymerized hydrogel protrude or migrate into the parent artery. Slight surface erosion defects of 2-10 μm in depth were observed after loading compared to 2 μm maximum for non-loaded hydrogels. These results show that our fine-tuned photopolymerized hydrogel is expected to withstand the physiological conditions of an in vivo implant study.
Keywords: erosion; fatigue; hydrogels; intracranial aneurysms; polyethylene glycol dimethacrylate; pulsatile fluid flow-induced loading.
Copyright © 2021 Poupart, Conti, Schmocker, Pancaldi, Moser, Nuss, Sakar, Dobrocky, Grützmacher, Mosimann and Pioletti.
Conflict of interest statement
AS was a shareholder at Lumendo SA to which the intellectual property was licensed. Lumendo SA was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- ASTM D412 (2006). Standard Test Methods for Vulcanized Rubber and Thermoplastic Elastomers-Tension. West Conshohocken, PA: ASTM International.
-
- Bai R., Yang J., Suo Z. (2019). Fatigue of hydrogels. Eur. J. Mech. A/Solids, 74, 337–3709. 10.1016/j.euromechsol.2018.12.001 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

