Thyroid Hormones Interaction With Immune Response, Inflammation and Non-thyroidal Illness Syndrome
- PMID: 33553149
- PMCID: PMC7859329
- DOI: 10.3389/fcell.2020.614030
Thyroid Hormones Interaction With Immune Response, Inflammation and Non-thyroidal Illness Syndrome
Abstract
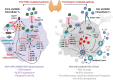
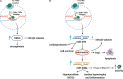
The interdependence between thyroid hormones (THs), namely, thyroxine and triiodothyronine, and immune system is nowadays well-recognized, although not yet fully explored. Synthesis, conversion to a bioactive form, and release of THs in the circulation are events tightly supervised by the hypothalamic-pituitary-thyroid (HPT) axis. Newly synthesized THs induce leukocyte proliferation, migration, release of cytokines, and antibody production, triggering an immune response against either sterile or microbial insults. However, chronic patho-physiological alterations of the immune system, such as infection and inflammation, affect HPT axis and, as a direct consequence, THs mechanism of action. Herein, we revise the bidirectional crosstalk between THs and immune cells, required for the proper immune system feedback response among diverse circumstances. Available circulating THs do traffic in two distinct ways depending on the metabolic condition. Mechanistically, internalized THs form a stable complex with their specific receptors, which, upon direct or indirect binding to DNA, triggers a genomic response by activating transcriptional factors, such as those belonging to the Wnt/β-catenin pathway. Alternatively, THs engage integrin αvβ3 receptor on cell membrane and trigger a non-genomic response, which can also signal to the nucleus. In addition, we highlight THs-dependent inflammasome complex modulation and describe new crucial pathways involved in microRNA regulation by THs, in physiological and patho-physiological conditions, which modify the HPT axis and THs performances. Finally, we focus on the non-thyroidal illness syndrome in which the HPT axis is altered and, in turn, affects circulating levels of active THs as reported in viral infections, particularly in immunocompromised patients infected with human immunodeficiency virus.
Keywords: Wnt/β-catenin; human immunodeficiency virus; hypothalamic–pituitary–thyroid; immune system; inflammasome; microRNAs; non-thyroidal illness syndrome; thyroid hormones.
Copyright © 2021 De Luca, Davis, Lin, Gionfra, Percario, Affabris, Pedersen, Marchese, Trivedi, Anastasiadou, Negro and Incerpi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Amores-Iniesta J., Barberà-Cremades M., Martiníz C. M., Pons J. A., Revilla-Nuin B., Martínez- Alarcón L., et al. (2017). Extracellular ATP activates the NLRP3 inflammasome and is an early danger signal of skin allograft rejection. Cell Rep. 21 3414–3426. 10.1016/j.celrep.2017.11.079 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

