Circular RNA CircCOL5A1 Sponges the MiR-7-5p/Epac1 Axis to Promote the Progression of Keloids Through Regulating PI3K/Akt Signaling Pathway
- PMID: 33553184
- PMCID: PMC7859531
- DOI: 10.3389/fcell.2021.626027
Circular RNA CircCOL5A1 Sponges the MiR-7-5p/Epac1 Axis to Promote the Progression of Keloids Through Regulating PI3K/Akt Signaling Pathway
Abstract
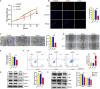
Keloids, as a result of abnormal wound healing in susceptible individuals, are characterized by the hyper-proliferation of fibroblasts and exaggerated deposition of extracellular matrix. Current surgical and therapeutic modalities provide limited satisfactory results. Growing evidence has highlighted the roles of circRNAs in acting as miRNA sponges. However, up to date, the regulatory mechanism of circRNAs in the pathological process of keloids has rarely been reported. In this study, cell proliferation, cell migration, flow cytometry, western blotting, fluorescence in situ hybridization, dual-luciferase activity, and immunohistochemistry assays were applied to explore the roles and mechanisms of the circCOL5A1/miR-7-5p/Epac1 axis in the keloid. The therapeutic potential of circCOL5A1 was investigated by establishing keloid implantation models. The RT-qPCR result revealed that circCOL5A1 expression was obviously higher in keloid tissues and keloid fibroblasts. Subsequent cellular experiments demonstrated that circCOL5A1 knockdown repressed the proliferation, migration, extracellular matrix (ECM) deposition, whereas promoted cell apoptosis, through the PI3K/Akt signaling pathway. Furthermore, RNA-fluorescence in situ hybridization (RNA-FISH) illustrated that both circCOL5A1 and miR-7-5p were located in the cytoplasm. The luciferase reporter gene assay confirmed that exact binding sites were present between circCOL5A1 and miR-7-5p, as well as between miR-7-5p and Epac1. Collectively, the present study revealed that circCOL5A1 functioned as competing endogenous RNA (ceRNA) by adsorbing miR-7-5p to release Epac1, which contributed to pathological hyperplasia of keloids through activating the PI3K/Akt signaling pathway. Our data indicated that circCOL5A1 might serve as a novel promising therapeutic target and represent a new avenue to understand underlying pathogenesis for keloids.
Keywords: Epac1; PI3K/Akt pathway; circCOL5A1; fibroblast; keloid; miR-7-5p.
Copyright © 2021 Lv, Liu, Zhang, Hu, Wu and Ren.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







References
-
- Christensen A. E., Selheim F., de Rooij J., Dremier S., Schwede F., Dao K. K., et al. (2003). cAMP analog mapping of Epac1 and cAMP kinase. Discriminating analogs demonstrate that Epac and cAMP kinase act synergistically to promote PC-12 cell neurite extension. J. Biol. Chem. 278 35394–35402. 10.1074/jbc.M302179200 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

