Ex Situ Dual Hypothermic Oxygenated Machine Perfusion for Human Split Liver Transplantation
- PMID: 33553615
- PMCID: PMC7862033
- DOI: 10.1097/TXD.0000000000001116
Ex Situ Dual Hypothermic Oxygenated Machine Perfusion for Human Split Liver Transplantation
Abstract
Liver splitting allows the opportunity to share a deceased graft between 2 recipients but remains underutilized. We hypothesized that liver splitting during continuous dual hypothermic oxygenated machine perfusion (DHOPE) is feasible, with shortened total cold ischemia times and improved logistics. Here, we describe a left lateral segment (LLS) and extended right lobe (ERL) liver split procedure during continuous DHOPE preservation with subsequent transplantation at 2 different centers.
Methods: After transport using static cold storage, a 51-year-old brain death donor liver underwent end-ischemic DHOPE. During DHOPE, the donor liver was maintained <10 °C and oxygenated with a Po2 of >106 kPa. An ex situ ERL/LLS split was performed with continuing DHOPE throughout the procedure to avoid additional ischemia time.
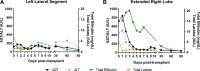
Results: Total cold ischemia times for the LLS and ERL were 205 minutes and 468 minutes, respectively. Both partial grafts were successfully transplanted at 2 different transplant centers. Peak aspartate aminotransferase and alanine aminotransferase were 172 IU/L and 107 IU/L for the LLS graft, and 839 IU/L and 502 IU/L for the ERL graft, respectively. The recipient of the LLS experienced an episode of acute cellular rejection. The ERL transplantation was complicated by severe acute pancreatitis with jejunum perforation requiring percutaneous drainage and acute cellular rejection. No device-related adverse events were observed.
Conclusions: Liver splitting during continuous DHOPE preservation is feasible, has the potential to substantially shorten cold ischemia time and may optimize transplant logistics. Therefore liver splitting with DHOPE can potentially improve utilization of split liver transplantation.
Copyright © 2021 The Author(s). Transplantation Direct. Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
The authors declare no funding or conflicts of interest.
Figures


References
-
- Pichlmayr R, Ringe B, Gubernatis G, et al. . [Transplantation of a donor liver to 2 recipients (splitting transplantation)–a new method in the further development of segmental liver transplantation]. Langenbecks Arch Chir. 1988; 373:127–130 - PubMed
-
- Rogiers X, Malago M, Habib N, et al. . In situ splitting of the liver in the heart-beating cadaveric organ donor for transplantation in two recipients. Transplantation. 1995; 59:1081–1083 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

