Cartilage-targeting peptide-modified dual-drug delivery nanoplatform with NIR laser response for osteoarthritis therapy
- PMID: 33553822
- PMCID: PMC7844135
- DOI: 10.1016/j.bioactmat.2021.01.017
Cartilage-targeting peptide-modified dual-drug delivery nanoplatform with NIR laser response for osteoarthritis therapy
Abstract
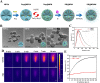
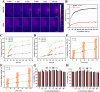
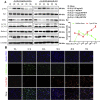
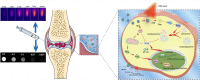
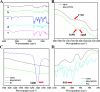
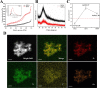
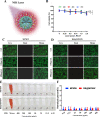
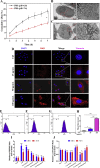
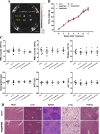
Cartilage-targeting delivery of therapeutic agents is still an effective strategy for osteoarthritis (OA) therapy. Recently, scavenging for reactive oxygen species (ROS) and activating autophagy have been increasingly reported to treat OA effectively. In this study, we designed, for the first time, a dual-drug delivery system based on metal organic framework (MOF)-decorated mesoporous polydopamine (MPDA) which composed of rapamycin (Rap) loaded into the mesopores and bilirubin (Br) loaded onto the shell of MOF. The collagen II-targeting peptide (WYRGRL) was then conjugated on the surface of above nanocarrier to develop a cartilage-targeting dual-drug delivery nanoplatform (RB@MPMW). Our results indicated the sequential release of two agents from RB@MPMW could be achieved via near-infrared (NIR) laser irritation. Briefly, the rapid release of Br from the MOF shell exhibited excellent ROS scavenging ability and anti-apoptosis effects, however responsively reduced autophagy activity, to a certain extent. Meanwhile, following the NIR irradiation, Rap was rapidly released from MPDA core and further enhanced autophagy activation and chondrocyte protection. RB@MPMW continuously phosphorylated AMPK and further rescued mitochondrial energy metabolism of chondrocytes following IL-1β stimulation via activating SIRT1-PGC-1α signaling pathway. Additionally, the cartilage-targeting property of peptide-modified nanocarrier could be monitored via Magnetic Resonance (MR) and IVIS imaging. More significantly, RB@MPMW effectively delayed cartilage degeneration in ACLT rat model. Overall, our findings indicated that the as-prepared dual-drug delivery nanoplatform exerted potent anti-inflammation and anti-apoptotic effects, rescued energy metabolism of chondrocytes in vitro and prevented cartilage degeneration in vivo, which thereby showed positive performance for OA therapy.
Keywords: Autophagy; Energy metabolism; Osteoarthritis; Oxidative stress; Targeting therapy.
© 2021 The Authors. Production and hosting by Elsevier B.V. on behalf of KeAi Communications Co., Ltd.
Conflict of interest statement
All authors declared that no conflict interest existed.
Figures








References
-
- Kloppenburg M., Berenbaum F. Osteoarthritis year in review 2019: epidemiology and therapy. Osteoarthritis Cartilage. 2020;28:242–248. - PubMed
-
- Nasiri N., Hosseini S., Alini M., Khademhosseini A., Baghaban Eslaminejad M. Targeted cell delivery for articular cartilage regeneration and osteoarthritis treatment, Drug Discov. Today Off. 2019;24:2212–2224. - PubMed
-
- da Costa B.R., Reichenbach S., Keller N., Nartey L., Wandel S., Juni P., Trelle S. Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta-analysis. Lancet. 2017;390:e21–e33. - PubMed
-
- Lepetsos P., Papavassiliou A.G. ROS/oxidative stress signaling in osteoarthritis. Biochim. Biophys. Acta. 2016;1862:576–591. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

