Phage-Display-Derived Peptide Specific to Carbamylated Protein
- PMID: 33553925
- PMCID: PMC7860060
- DOI: 10.1021/acsomega.0c05481
Phage-Display-Derived Peptide Specific to Carbamylated Protein
Abstract
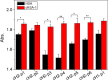
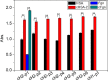
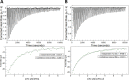
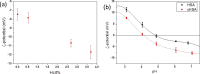
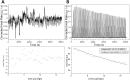
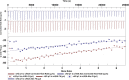
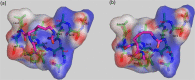
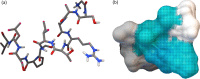
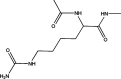
Protein carbamylation has been linked with diseases commonly associated with patients with reduced kidney function. Carbamylated human serum albumin (cHSA), which has been proven to be nephrotoxic and associated with heart failure for chronic kidney disease (CKD) patients, was chosen for our study. Through phage display against cHSA, one specific peptide sequence (cH2-p1) was identified with higher selectivity toward cHSA over native HSA. The cH2-p1 peptide was synthesized, and its target binding was analyzed through isothermal titration calorimetry (ITC). The result showed that cH2-p1 was able to bind cHSA of different levels of carbamylation with a similar dissociation constant of ∼1.0 × 10-4 M. This peptide also showed a binding specificity to carbamylated fibrinogen (cFgn), while not binding to native Fgn at all. For better understanding of the binding mechanism of cH2-p1, competitive binding of cH2-p1 and anti-homocitrulline to cHSA was performed, and the result revealed that cH2-p1 may bind to homocitrulline residues in a similar manner to the antibody. A molecular docking study was further performed to investigate the favored binding conformation of homocitrulline residue to cH2-p1. This work demonstrates the potential of peptides as a specific binding element to carbamylated proteins.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Fazili K. M.; Mir M. M.; Qasim M. A. Changes in Protein Stability Upon Chemical Modification of Lysine Residues of Bovine Serum-Albumin by Different Reagents. Biochem. Mol. Biol. Int. 1993, 31, 807–816. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

