Fecal Microbiota Transplant in Cirrhosis Reduces Gut Microbial Antibiotic Resistance Genes: Analysis of Two Trials
- PMID: 33553973
- PMCID: PMC7850310
- DOI: 10.1002/hep4.1639
Fecal Microbiota Transplant in Cirrhosis Reduces Gut Microbial Antibiotic Resistance Genes: Analysis of Two Trials
Abstract
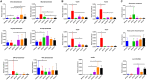
Antibiotic resistance leads to poor outcomes in cirrhosis. Fecal microbiota transplant (FMT) is associated with reduction in antibiotic resistance gene (ARG) burden in patients without cirrhosis; however, the impact in cirrhosis is unclear. We aimed to study the effect of capsule and enema FMT on ARG abundance in fecal samples, which were collected during two published FMT trials in patients with cirrhosis on rifaximin, lactulose, and proton pump inhibitors. ARGs were identified using metagenomics and mapped against the Comprehensive Antibiotic Resistance Database. Changes in ARG abundance were studied within/between groups. The capsule FMT trial involved a one-time FMT or placebo capsule administration with stool collection at baseline and week 4 postintervention. Antibiotics+enema FMT included preprocedure antibiotics followed by FMT enema versus standard-of-care (SOC). Stool was collected at baseline, postantibiotics, and day 7/15 postintervention. Both trials included 20 patients each. There was no safety/infection signal linked to FMT. In the capsule trial, beta-lactamase (OXY/LEN) expression decreased post-FMT versus baseline. Compared to placebo, patients who were post-FMT had lower abundance of vancomycin (VanH), beta-lactamase (ACT), and rifamycin ARGs; the latter was associated with cognitive improvement. No changes were seen within patients treated with placebo. In the antibiotics+enema trial for postantibiotics at day 7 versus baseline, there was an increase in vancomycin and beta-lactamase ARGs, which decreased at day 15. However, quinolone resistance increased at day 15 versus baseline. Between SOC and FMT, day 7 had largely lower ARG (CfxA beta-lactamase, VanW, and VanX) that continued at day 15 (cepA beta-lactamase, VanW). No changes were seen within the SOC group. Conclusion: Despite differences in routes of administration and preintervention antibiotics, we found that ARG abundance is largely reduced after FMT compared to pre-FMT baseline and non-FMT groups in decompensated cirrhosis.
© 2020 The Authors. Hepatology Communications published by Wiley Periodicals LLC on behalf of the American Association for the Study of Liver Diseases.
Figures





References
-
- Fernandez J, Prado V, Trebicka J, Amoros A, Gustot T, Wiest R, et al.; European Foundation for the Study of Chronic Liver Failure (EF‐Clif) . Multidrug‐resistant bacterial infections in patients with decompensated cirrhosis and with acute‐on‐chronic liver failure in Europe. J Hepatol 2019;70:398‐411. - PubMed
-
- Martin M. Cutadapt removes adapter sequences from high‐throughput sequencing reads. Embnet.J 2011;17 10.14806/ej.17.1.200. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

