Effect of Personalized Breast Cancer Risk Tool on Chemoprevention and Breast Imaging: ENGAGED-2 Trial
- PMID: 33554037
- PMCID: PMC7853161
- DOI: 10.1093/jncics/pkaa114
Effect of Personalized Breast Cancer Risk Tool on Chemoprevention and Breast Imaging: ENGAGED-2 Trial
Abstract
Background: Limited evidence exists about how to communicate breast density-informed breast cancer risk to women at elevated risk to motivate cancer prevention.
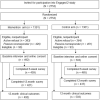
Methods: We conducted a randomized controlled trial evaluating a web-based intervention incorporating personalized breast cancer risk, information on chemoprevention, and values clarification on chemoprevention uptake vs active control. Eligible women aged 40-69 years with normal mammograms and elevated 5-year breast cancer risk were recruited from Kaiser Permanente Washington from February 2017 to May 2018. Chemoprevention uptake was measured as any prescription for raloxifene or tamoxifen within 12 months from baseline in electronic health record pharmacy data. Secondary outcomes included breast magnetic resonance imaging (MRI), mammography use, self-reported distress, and communication with providers. We calculated unadjusted odds ratios (ORs) using logistic regression models and mean differences using analysis of covariance models with 95% confidence intervals (CIs) with generalized estimating equations.
Results: We randomly assigned 995 women to the intervention arm (n = 492) or control arm (n = 503). The intervention (vs control) had no effect on chemoprevention uptake (OR = 1.04, 95% CI = 0.07 to 16.62). The intervention increased breast MRI use (OR = 5.65, 95% CI = 1.61 to 19.74) while maintaining annual mammography (OR = 0.98, 95% CI = 0.75 to 1.28). Women in the intervention (vs control) arm had 5.67-times higher odds of having discussed chemoprevention or breast MRI with provider by 6 weeks (OR = 5.67, 95% CI = 2.47 to 13.03) and 2.36-times higher odds by 12 months (OR = 2.36, 95% CI = 1.65 to 3.37). No measurable differences in distress were detected.
Conclusions: A web-based, patient-level intervention activated women at elevated 5-year breast cancer risk to engage in clinical discussions about chemoprevention, but uptake remained low.
© The Author(s) 2021. Published by Oxford University Press.
Figures
Similar articles
-
Effect of a Randomized Trial of a Web-Based Intervention on Patient-Provider Communication About Breast Density.J Womens Health (Larchmt). 2021 Nov;30(11):1529-1537. doi: 10.1089/jwh.2021.0053. Epub 2021 Sep 28. J Womens Health (Larchmt). 2021. PMID: 34582721 Free PMC article. Clinical Trial.
-
A web-based personalized risk communication and decision-making tool for women with dense breasts: Design and methods of a randomized controlled trial within an integrated health care system.Contemp Clin Trials. 2017 May;56:25-33. doi: 10.1016/j.cct.2017.02.009. Epub 2017 Feb 28. Contemp Clin Trials. 2017. PMID: 28257920 Free PMC article. Clinical Trial.
-
Use of tamoxifen and raloxifene for breast cancer chemoprevention in 2010.Breast Cancer Res Treat. 2012 Jul;134(2):875-80. doi: 10.1007/s10549-012-2089-2. Epub 2012 May 24. Breast Cancer Res Treat. 2012. PMID: 22622807 Free PMC article.
-
Carcinoma in situ outcomes in National Surgical Adjuvant Breast and Bowel Project Breast Cancer Chemoprevention Trials.J Natl Cancer Inst Monogr. 2010;2010(41):181-6. doi: 10.1093/jncimonographs/lgq041. J Natl Cancer Inst Monogr. 2010. PMID: 20956826 Free PMC article. Review.
-
Chemoprevention of breast cancer: a summary of the evidence for the U.S. Preventive Services Task Force.Ann Intern Med. 2002 Jul 2;137(1):59-69. doi: 10.7326/0003-4819-137-1-200207020-00017. Ann Intern Med. 2002. PMID: 12093250 Review.
Cited by
-
Estrogen Receptor Signaling in Breast Cancer.Cancers (Basel). 2023 Sep 23;15(19):4689. doi: 10.3390/cancers15194689. Cancers (Basel). 2023. PMID: 37835383 Free PMC article. Review.
-
Effect of a Randomized Trial of a Web-Based Intervention on Patient-Provider Communication About Breast Density.J Womens Health (Larchmt). 2021 Nov;30(11):1529-1537. doi: 10.1089/jwh.2021.0053. Epub 2021 Sep 28. J Womens Health (Larchmt). 2021. PMID: 34582721 Free PMC article. Clinical Trial.
-
A systematic review on eHealth technology personalization approaches.iScience. 2024 Aug 19;27(9):110771. doi: 10.1016/j.isci.2024.110771. eCollection 2024 Sep 20. iScience. 2024. PMID: 39290843 Free PMC article.
-
"I Thought Cancer was a Tobacco Issue": Perspectives of Veterans with and without HIV on Cancer and Other Health Risks Associated with Alcohol and Tobacco/Nicotine Use.AIDS Behav. 2024 Aug;28(8):2607-2618. doi: 10.1007/s10461-024-04363-6. Epub 2024 Jun 13. AIDS Behav. 2024. PMID: 38869757 Free PMC article.
References
-
- Kinsinger LS, Harris R, Woolf SH, Sox HC, Lohr KN.. Chemoprevention of breast cancer: a summary of the evidence for the US Preventive Services Task Force. Ann Intern Med. 2002;137(1):59-69. - PubMed
-
- Nelson HD, Fu R, Humphrey L, Smith B, Griffin JC, Nygren P. Comparative effectiveness of medications to reduce risk of primary breast cancer in women. Ann Intern Med. 2009;151(10):703-15, W-226-35. - PubMed
-
- Nelson HD, Fu R, Zakher B, Pappas M, McDonagh M.. Medication use for the risk reduction of primary breast cancer in women: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2019;322(9):868-886. - PubMed
-
- Owens DK, Davidson KW, Krist AH, et al.; US Preventive Services Task Force. Medication use to reduce risk of breast cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;322(9):857-867. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical