Multiple Myeloma: EHA-ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-up
- PMID: 33554050
- PMCID: PMC7861652
- DOI: 10.1097/HS9.0000000000000528
Multiple Myeloma: EHA-ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-up
Erratum in
-
Correction Notice: Multiple Myeloma: EHA-ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-up.Hemasphere. 2021 Mar 31;5(4):e567. doi: 10.1097/HS9.0000000000000567. eCollection 2021 Apr. Hemasphere. 2021. PMID: 33824948 Free PMC article.
-
Correction Notice: Multiple Myeloma: EHA-ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-up.Hemasphere. 2021 Nov 29;5(12):e659. doi: 10.1097/HS9.0000000000000659. eCollection 2021 Dec. Hemasphere. 2021. PMID: 34859196 Free PMC article.
Conflict of interest statement
MAD reported consultancy and honoraria from Janssen, Celgene, Takeda, Amgen and Bristol Myers Squibb. PM reported honoraria from Celgene, Janssen, Takeda, Amgen and Abbvie. ET reported honoraria from Bristol Myers Squibb, Janssen, Celgene, Takeda, Genesis Pharma, Amgen, Sanofi and Novartis and research funding from Janssen, Amgen, Takeda, Sanofi and Genesis Pharma. MVM reported honoraria from lectures and boards from Janssen, Celgene, Amgen, Takeda, Abbvie, GlaxoSmithKline, Adaptive, Roche and Seattle Genetics. SZ reported participation in advisory boards for Takeda, Celgene, Janssen, Sanofi and Oncopeptides and research funding from Celgene, Janssen and Takeda. GC reported being a member of speaker bureau for Takeda, Bristol Myers Squibb, Celgene, Amgen, Sanofi and Janssen and has received research grants from Bristol Myers Squibb, Celgene and Takeda. MD reported honoraria from Abbvie, Adaptive Biotechnologies, Amgen, Bristol Myers Squibb, Celgene, Janssen, Karyopharm, Sanofi and Takeda and has received research funding from Bristol Myers Squibb, Celgene, Janssen and Takeda. RH reported consultant or advisory roles for Janssen, Amgen, Celgene, AbbVie, Bristol Myers Squibb, Novartis, PharmaMar and Takeda; honoraria from Janssen, Amgen, Celgene, Bristol Myers Squibb, PharmaMar and Takeda and has received research grants from Janssen, Amgen, Celgene, Bristol Myers Squibb, Novartis and Takeda. FS reported honoraria from Amgen, Celgene, Bristol Myers Squibb, Takeda, Abbvie, Janssen, Novartis, SkyliteDX, Oncopeptides, Sanofi, GlaxoSmithKline, Adaptive and Merck Sharp & Dohme. MC reported honoraria from Janssen, Celgene, Amgen, Bristol Myers Squibb, Takeda, AbbVie, Sanofi and Adaptive Biotechnologies and speaker’s bureau membership for Janssen and Celgene. HG reported grants from Amgen, Bristol Myers Squibb, Celgene, Chugai, Dietmar-Hopp-Foundation, Janssen, John Hopkins University and Sanofi; research support from Amgen, Bristol Myers Squibb, Celgene, Chugai, Janssen, Incyte, Molecular Partners, Merck Sharp & Dohme, Sanofi, Mundipharma, Takeda and Novartis; participation in advisory boards for Adaptive Biotechnology, Amgen, Bristol Myers Squibb, Celgene, Janssen, Sanofi, Takeda; honoraria from Academy2 GmbH & Co. KG, Amgen, ArtTempi, Bristol Myers Squibb, Celgene, Chop GmbH, Chugai, FomF GmbH, GlaxoSmithKline, GWT Forschung und Innovation Dresden, InVo Institut für Versorgungsforschung in der Onkologie GbR, Janssen, Kompetenznetz Maligne Lymphome (KML), MedConcept GmbH, Medical Communication GmbH, New Concept Oncology, Novartis, Omnia Med Deutschland, Sanofi. TF reported speaker and advisory roles for Janssen, Bristol Myers Squibb, Takeda, advisory role for Roche, Sanofi, Karyopharm and Oncopeptides and Speaker for Amgen. HE reported consulting and advisory roles for Bristol Myers Squibb, Celgene, Janssen, Amgen, Takeda, Sanofi and GlaxoSmithKline; research funding from Bristol Myers Squibb, Celgene, Janssen, Amgen, GlaxoSmithKline and Sanofi; honoraria from Bristol Myers Squibb, Celgene, Amgen, Takeda, Sanofi and GlaxoSmithKline. MB has received honoraria from Sanofi, Celgene, Amgen, Janssen, Novartis, Bristol Myers Squibb and AbbVie; has served on the advisory boards for Janssen and GlaxoSmithKline; has received research funding from Sanofi, Celgene, Amgen, Janssen, Novartis, Bristol Myers Squibb and Mundipharma. JS-M reported consultancy for Amgen, Bristol Myers Squibb, Celgene, Janssen, Merck Sharp & Dohme, Novartis, GlaxoSmithKline, Takeda, Sanofi and Roche. PS reported honoraria and advisory roles for Celgene, Janssen, Amgen, Takeda, Bristol Myers Squibb and Skyline and research funding from Celgene, Amgen, Janssen and Takeda. UM reported honoraria from Celgene, Janssen, Amgen, Takeda, AbbVie, Bristol Myers Squibb and Sanofi.
Figures
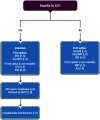
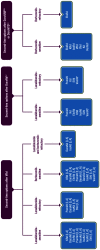
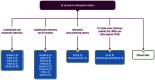
References
-
- Moreau P, San Miguel J, Sonneveld P, et al. Multiple myeloma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017; 28suppl_4iv52–iv61 - PubMed
-
- Kumar S, Paiva B, Anderson KC, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016; 17:e328–e346 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
