Isoform-selective decrease of glycogen synthase kinase-3-beta (GSK-3β) reduces synaptic tau phosphorylation, transcellular spreading, and aggregation
- PMID: 33554064
- PMCID: PMC7848608
- DOI: 10.1016/j.isci.2021.102058
Isoform-selective decrease of glycogen synthase kinase-3-beta (GSK-3β) reduces synaptic tau phosphorylation, transcellular spreading, and aggregation
Abstract
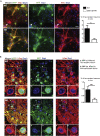
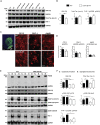
It has been suggested that aberrant activation of glycogen synthase kinase-3-beta (GSK-3β) can trigger abnormal tau hyperphosphorylation and aggregation, which ultimately leads to neuronal/synaptic damage and impaired cognition in Alzheimer disease (AD). We examined if isoform-selective partial reduction of GSK-3β can decrease pathological tau changes, including hyperphosphorylation, aggregation, and spreading, in mice with localized human wild-type tau (hTau) expression in the brain. We used adeno-associated viruses (AAVs) to express hTau locally in the entorhinal cortex of wild-type and GSK-3β hemi-knockout (GSK-3β-HK) mice. GSK-3β-HK mice had significantly less accumulation of hyperphosphorylated tau in synapses and showed a significant decrease of tau protein spread between neurons. In primary neuronal cultures from GSK-3β-HK mice, the aggregation of exogenous FTD-mutant tau was also significantly reduced. These results show that a partial decrease of GSK-3β significantly represses tau-initiated neurodegenerative changes in the brain, and therefore is a promising therapeutic target for AD and other tauopathies.
Keywords: Biological Sciences; Cellular Neuroscience; Neuroscience.
© 2021 The Authors.
Conflict of interest statement
Teresa Gómez-Isla participated as speaker in an Eli Lilly and Company-sponsored educational symposium and serves in an Eli Lilly Data Monitoring Committee. All other authors declare no competing interests.
Figures





References
-
- Arvanitakis Z., Witte R.J., Dickson D.W., Tsuboi Y., Uitti R.J., Slowinski J., Hutton M.L., Lin S.-C., Boeve B.F., Cheshire W.P. Clinical-pathologic study of biomarkers in FTDP-17 (PPND family with N279K tau mutation) Parkinsonism Relat. Disord. 2007;13:230–239. - PubMed
-
- Augustinack J.C., Schneider A., Mandelkow E.-M., Hyman B.T. Specific tau phosphorylation sites correlate with severity of neuronal cytopathology in Alzheimer’s disease. Acta Neuropathol. 2002;103:26–35. - PubMed
-
- Avila J., Lucas J.J., Pérez M., Hernández F. Role of tau protein in both physiological and pathological conditions. Physiol. Rev. 2004;84:361–384. - PubMed
-
- de Barreda E.G., Pérez M., Ramos P.G., de Cristobal J., Martín-Maestro P., Morán A., Dawson H.N., Vitek M.P., Lucas J.J., Hernández F. Tau-knockout mice show reduced GSK3-induced hippocampal degeneration and learning deficits. Neurobiol. Dis. 2010;37:622–629. - PubMed
-
- Bhat R.V., Andersson U., Andersson S., Knerr L., Bauer U., Sundgren-Andersson A.K. The conundrum of GSK3 inhibitors: is it the dawn of a new beginning? J. Alzheimers Dis. 2018;64:S547–S554. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

