A global point prevalence survey of antimicrobial use in neonatal intensive care units: The no-more-antibiotics and resistance (NO-MAS-R) study
- PMID: 33554094
- PMCID: PMC7848759
- DOI: 10.1016/j.eclinm.2021.100727
A global point prevalence survey of antimicrobial use in neonatal intensive care units: The no-more-antibiotics and resistance (NO-MAS-R) study
Abstract
Background: Global assessment of antimicrobial agents prescribed to infants in the neonatal intensive care unit (NICU) may inform antimicrobial stewardship efforts.
Methods: We conducted a one-day global point prevalence study of all antimicrobials provided to NICU infants. Demographic, clinical, and microbiologic data were obtained including NICU level, census, birth weight, gestational/chronologic age, diagnoses, antimicrobial therapy (reason for use; length of therapy), antimicrobial stewardship program (ASP), and 30-day in-hospital mortality.
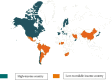
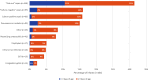
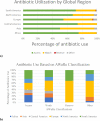
Findings: On July 1, 2019, 26% of infants (580/2,265; range, 0-100%; median gestational age, 33 weeks; median birth weight, 1800 g) in 84 NICUs (51, high-income; 33, low-to-middle income) from 29 countries (14, high-income; 15, low-to-middle income) in five continents received ≥1 antimicrobial agent (92%, antibacterial; 19%, antifungal; 4%, antiviral). The most common reasons for antibiotic therapy were "rule-out" sepsis (32%) and "culture-negative" sepsis (16%) with ampicillin (40%), gentamicin (35%), amikacin (19%), vancomycin (15%), and meropenem (9%) used most frequently. For definitive treatment of presumed/confirmed infection, vancomycin (26%), amikacin (20%), and meropenem (16%) were the most prescribed agents. Length of therapy for culture-positive and "culture-negative" infections was 12 days (median; IQR, 8-14) and 7 days (median; IQR, 5-10), respectively. Mortality was 6% (42%, infection-related). An NICU ASP was associated with lower rate of antibiotic utilization (p = 0·02).
Interpretation: Global NICU antibiotic use was frequent and prolonged regardless of culture results. NICU-specific ASPs were associated with lower antibiotic utilization rates, suggesting the need for their implementation worldwide.
Funding: Merck & Co.; The Ohio State University College of Medicine Barnes Medical Student Research Scholarship.
Keywords: Antibiotics; Antifungal; Global point prevalence study; Neonatal antimicrobial stewardship; Neonatal infection.
© 2021 The Authors.
Conflict of interest statement
Dr. Pablo J. Sánchez has received research grant support from Merck & Co. during the conduct of the study, and grant from MedImmune, Inc - AstraZeneca, outside of the submitted work. Dr. Pavel Prusakov has received research grant support from Merck & Co. and Pfizer. Dr. Debra A. Goff has received research grant support from Merck & Co. and Pfizer. Dr. Landgrave reports other support from GSK, outside the submitted work. Dr. Kekomäki reports grants and personal fees from Sanofi, grants and personal fees from Merck Sharp & Dome, other support from Pfizer, all outside of the submitted work. Dr. Mesa reports speaker fees from Pfizer and GlaxoSmithKline, outside of the submitted work. Mr. Wozniak received a Barnes Medical Student Research Scholarship grant from The Ohio State University College of Medicine. The other authors have nothing to disclose.
Figures
References
-
- Grohskopf L.A., Huskins W.C., Sinkowitz-Cochran R.L., Levine G.L., Goldmann D.A., Jarvis W.R. Use of antimicrobial agents in United States neonatal and pediatric intensive care patients. Pediatr Infect Dis J. 2005;24(9):766–773. - PubMed
-
- Shane A.L., Sanchez P.J., Stoll B.J. Neonatal sepsis. Lancet. 2017;390(10104):1770–1780. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

