Imaging the early zebrafish embryo centrosomes following injection of small-molecule inhibitors to understand spindle formation
- PMID: 33554134
- PMCID: PMC7843657
- DOI: 10.1016/j.xpro.2020.100293
Imaging the early zebrafish embryo centrosomes following injection of small-molecule inhibitors to understand spindle formation
Abstract
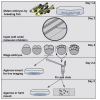
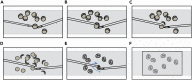
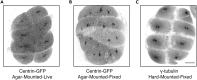
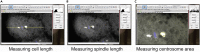
During the earliest division stages, zebrafish embryos have large cells that divide rapidly and synchronously to create a cellular layer on top of the yolk. Here, we describe a protocol for monitoring spindle dynamics during these early embryonic divisions. We outline techniques for injecting zebrafish embryos with small-molecule inhibitors toward polo-like kinases, preparing and mounting embryos for three-dimensional imaging using confocal microscopy. These techniques are used to understand how the early zebrafish embryo's centrosome constructs the mitotic spindle. For complete details on the use and execution of this protocol, please refer to Rathbun et al. (2020).
Keywords: Cell biology; Microscopy; Model organisms.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Kimmel C.B., Ballard W.W., Kimmel S.R., Ullmann B., Schilling T.F. Stages of embryonic development of the Zebrafish. Dev. Dyn. 1995;203:253–310. - PubMed
-
- JoVE Science Education Database . JoVE; 2020. Biology II: Mouse, Zebrafish, and Chick. Zebrafish Microinjection Techniques.
-
- Westerfield M. Fourth Edition. University of Oregon Press; 2000. The zebrafish book. A guide for the laboratory use of zebrafish (Danio rerio)
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

