Protocol for the separation of extracellular vesicles by ultracentrifugation from in vitro cell culture models
- PMID: 33554138
- PMCID: PMC7848770
- DOI: 10.1016/j.xpro.2021.100303
Protocol for the separation of extracellular vesicles by ultracentrifugation from in vitro cell culture models
Abstract
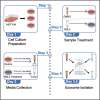
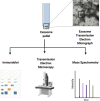
Extracellular vesicles (EVs) play key roles in transporting key molecular constituents as cargo for extracellular trafficking. While several approaches have been developed to extract EVs from mammalian cells, the specific method of EV isolation can have a profound effect on membrane integrity and yield. Here, we describe a step-by-step procedure to separate EVs from adherent epithelial cells using differential ultracentrifugation. Separated EVs can be further analyzed by immunoblotting, mass spectrometry, and transmission electron microscopy to derive EV yield and morphology. For complete details on the use and execution of this protocol, please refer to Brown et al. (2019).
Keywords: Cancer; Cell biology; Cell culture; Cell membrane.
© 2021 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Coumans F.A.W., Brisson A.R., Buzas E.I., Dignat-George F., Drees E.E.E., El-Andaloussi S., Emanueli C., Gasecka A., Hendrix A., Hill A.F. Methodological guidelines to study extracellular vesicles. Circ. Res. 2017;120:1632–1648. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

