Survival and Causes of Death Among Veterans With Lower Extremity Revascularization With Paclitaxel-Coated Devices: Insights From the Veterans Health Administration
- PMID: 33554613
- PMCID: PMC7955346
- DOI: 10.1161/JAHA.120.018149
Survival and Causes of Death Among Veterans With Lower Extremity Revascularization With Paclitaxel-Coated Devices: Insights From the Veterans Health Administration
Abstract
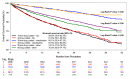
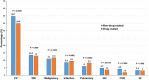
BACKGROUND The long-term safety of paclitaxel-coated devices (PCDs; drug-coated balloon or drug-eluting stent) for peripheral endovascular intervention is uncertain. We used data from the Veterans Health Administration to evaluate the association between PCDs, long-term mortality, and cause of death. METHODS AND RESULTS Using the Veterans Administration Corporate Data Warehouse in conjunction with International Classification of Diseases, Tenth Revision (ICD-10) Procedure Coding System, Current Procedural Terminology, and Healthcare Common Procedure Coding System codes, we identified patients with peripheral artery disease treated within the Veterans Administration for femoropopliteal artery revascularization between October 1, 2015, and June 30, 2019. An adjusted Cox regression, using stabilized inverse probability-weighted estimates, was used to evaluate the association between PCDs and long-term survival. Cause of death data were obtained using the National Death Index. In total, 10 505 patients underwent femoropopliteal peripheral endovascular intervention; 2265 (21.6%) with a PCD and 8240 (78.4%) with a non-PCD (percutaneous angioplasty balloon and/or bare metal stent). Survival rates at 2 years (77.4% versus 79.7%) and 3 years (70.7% versus 71.8%) were similar between PCD and non-PCD groups, respectively. The adjusted hazard for all-cause mortality for patients treated with a PCD versus non-PCD was 1.06 (95% CI, 0.95-1.18, P=0.3013). Among patients who died between October 1, 2015, and December 31, 2017, the cause of death according to treatment group, PCD versus non-PCD, was similar. CONCLUSIONS Among patients undergoing femoropopliteal peripheral endovascular intervention within the Veterans Administration Health Administration, there was no increased risk of long-term, all-cause mortality associated with PCD use. Cause-specific mortality rates were similar between treatment groups.
Keywords: paclitaxel; peripheral artery disease; peripheral endovascular intervention.
Conflict of interest statement
Dr. Gutierrez discloses the following relationships: a Veterans Health Administration Career Development Award and consulting for Janssen Pharmaceuticals and Amgen Inc. Dr. Swaminathan discloses the following relationships: research support from ACIST Medical, site principal investigator for Cardiovascular Systems Inc., and consulting for Medtronic. Dr. Schuyler Jones discloses research support from Medtronic. Dr. Secemsky discloses the following relationships: research grants to Beth Israel Deaconess Medical Center, AstraZeneca, Becton Dickinson Bard Medical, Boston Scientific, Cook Medical, CSI, Medtronic, Philips, and University of California San Francisco and consulting for Becton Dickinson Bard Medical, Cook Medical, CSI, Janssen, Medtronic, and Philips. Dr. Armstrong discloses the following relationships: consultant to Abbott Vascular, Boston Scientific, Cardiovascular Systems, Gore, Intact Vascular, Medtronic, Philips, and PQ Bypass. The remaining authors have no disclosures to report.
Figures

Comment in
-
Paclitaxel-Coated Devices: Safety and Efficacy Are in the PVI of the Beholder.J Am Heart Assoc. 2021 Feb 16;10(4):e020289. doi: 10.1161/JAHA.120.020289. Epub 2021 Feb 6. J Am Heart Assoc. 2021. PMID: 33554617 Free PMC article. No abstract available.
References
-
- Katsanos K, Spiliopoulos S, Kitrou P, Krokidis M, Karnabatidis D. Risk of death following application of paclitaxel‐coated balloons and stents in the femoropopliteal artery of the leg: a systematic review and meta‐analysis of randomized controlled trials. J Am Heart Assoc. 2018;7:e011245. DOI: 10.1161/JAHA.118.011245 . - DOI - PMC - PubMed
-
- Velarde KE, Romesser JM, Johnson MR, Clegg DO, Efimova O, Oostema SJ, Scehnet JS, DuVall SL, Huang GD. An initiative using informatics to facilitate clinical research planning and recruitment in the VA health care system. Contemp Clin Trials Commun. 2018;11:107–112. DOI: 10.1016/j.conctc.2018.07.001 . - DOI - PMC - PubMed
-
- Maynard C, Trivedi R, Nelson K, Fihn SD. Disability rating, age at death, and cause of death in U.S. veterans with service‐connected conditions. Mil Med. 2018;183:e371–e376. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

