Characterization and Functional Analysis of CD44v6.CAR T Cells Endowed with a New Low-Affinity Nerve Growth Factor Receptor-Based Spacer
- PMID: 33554732
- PMCID: PMC8312023
- DOI: 10.1089/hum.2020.216
Characterization and Functional Analysis of CD44v6.CAR T Cells Endowed with a New Low-Affinity Nerve Growth Factor Receptor-Based Spacer
Abstract
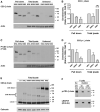
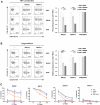
Effectiveness of adoptively transferred chimeric antigen receptor (CAR) T cells strongly depends on the quality of CAR-mediated interaction of the effector cells with the target antigen on tumor cells. A major role in this interaction is played by the affinity of the single-chain variable fragment (scFv) for the antigen, and by the CAR design. In particular, the spacer domain may impact on the CAR T cell function by affecting the length and flexibility of the resulting CAR. This study addresses the need to improve the manufacturing process and the antitumor activity of CD44v6-specific CAR T cells by defining the optimal structure of a spacer region derived from the extracellular domain of the human low-affinity nerve growth factor receptor (LNGFR). We tailored the LNGFR spacer to modulate CAR length to efficiently recognize distal or proximal epitopes and to allow selection of transduced CAR T cells by the use of clinical-grade validated manufacturing systems. The different LNGFR spacers investigated in this study are responsible for the generation of CAR T cells with a different memory phenotype, which is mainly related to the level of CAR expression and the extent of the associated tonic signaling. In particular, the CD44v6-NWN2.CAR T cells are enriched in central memory cells and show improved in vitro functions in terms of killing capability, and in vivo antitumor activity against hematological and solid tumors. Clinical Trial Registration numbers: clinicaltrial.gov NCT04097301; ClinicalTrials.gov, NCT00423124.
Keywords: CAR T; CD44v6; adoptive cell therapy; solid tumor; spacer.
Conflict of interest statement
C.S., C.A., and S.P. were MolMed employees. A.S., B.V., C.S., D.Z., Y.M.D.L.T., S.C., and C.T. are AGC Biologics SpA (formerly MolMed SpA) employees. AGC Biologics SpA (formerly MolMed SpA) is the applicant of patents on CAR molecules containing LNGFR-derived spacers including the CD44v6-targeted CARs studied in this work.
Figures






References
-
- Sadelain M, Riviere I, Brentjens R. Targeting tumours with genetically enhanced T lymphocytes. Nat Rev Cancer 2003;3:35–45 - PubMed
-
- Ho WY, Blattman JN, Dossett ML, et al. Adoptive immunotherapy: engineering T cell responses as biologic weapons for tumor mass destruction. Cancer Cell 2003;3:431–437 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

