Divergent, Strain-Release Reactions of Azabicyclo[1.1.0]butyl Carbinols: Semipinacol or Spiroepoxy Azetidine Formation
- PMID: 33555105
- PMCID: PMC8247891
- DOI: 10.1002/anie.202100583
Divergent, Strain-Release Reactions of Azabicyclo[1.1.0]butyl Carbinols: Semipinacol or Spiroepoxy Azetidine Formation
Abstract
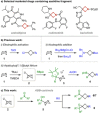
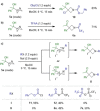
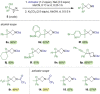
The azetidine moiety is a privileged motif in medicinal chemistry and new methods that access them efficiently are highly sought after. Towards this goal, we have found that azabicyclo[1.1.0]butyl carbinols, readily obtained from the highly strained azabicyclo[1.1.0]butane (ABB), can undergo divergent strain-release reactions upon N-activation. Treatment with trifluoroacetic anhydride or triflic anhydride triggered a semipinacol rearrangement to give keto 1,3,3-substituted azetidines. More than 20 examples were explored, enabling us to evaluate selectivity and the migratory aptitude of different groups. Alternatively, treatment of the same alcohols with benzyl chloroformate in the presence of NaI led to iodohydrin intermediates which gave spiroepoxy azetidines upon treatment with base. The electronic nature of the activating agent dictates which pathway operates.
Keywords: azabicyclo[1.1.0]butane; azetidines; epoxides; ring expansion; strained molecules.
© 2021 Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Synthesis of 2-(Trifluoromethyl)Azetidines by Strain-Release Reactions of 2-(Trifluoromethyl)-1-Azabicyclo[1.1.0]Butanes.Chemistry. 2025 May 22;31(29):e202500590. doi: 10.1002/chem.202500590. Epub 2025 Apr 21. Chemistry. 2025. PMID: 40193205 Free PMC article.
-
Synthesis and Applications of Bicyclo[1.1.0]butyl and Azabicyclo[1.1.0]butyl Organometallics.Chemistry. 2023 May 22;29(29):e202300008. doi: 10.1002/chem.202300008. Epub 2023 Apr 14. Chemistry. 2023. PMID: 36786481 Free PMC article. Review.
-
Strain-Release Driven Spirocyclization of Azabicyclo[1.1.0]butyl Ketones.Angew Chem Int Ed Engl. 2021 May 17;60(21):11824-11829. doi: 10.1002/anie.202102754. Epub 2021 Apr 16. Angew Chem Int Ed Engl. 2021. PMID: 33754432 Free PMC article.
-
Four-Component Strain-Release-Driven Synthesis of Functionalized Azetidines.Angew Chem Int Ed Engl. 2022 Dec 23;61(52):e202214049. doi: 10.1002/anie.202214049. Epub 2022 Nov 27. Angew Chem Int Ed Engl. 2022. PMID: 36300572 Free PMC article.
-
The renaissance of strained 1-azabicyclo[1.1.0]butanes as useful reagents for the synthesis of functionalized azetidines.Org Biomol Chem. 2020 Aug 5;18(30):5798-5810. doi: 10.1039/d0ob01251c. Org Biomol Chem. 2020. PMID: 32687133 Review.
Cited by
-
Synthesis of 2-(Trifluoromethyl)Azetidines by Strain-Release Reactions of 2-(Trifluoromethyl)-1-Azabicyclo[1.1.0]Butanes.Chemistry. 2025 May 22;31(29):e202500590. doi: 10.1002/chem.202500590. Epub 2025 Apr 21. Chemistry. 2025. PMID: 40193205 Free PMC article.
-
Synthesis and Applications of Bicyclo[1.1.0]butyl and Azabicyclo[1.1.0]butyl Organometallics.Chemistry. 2023 May 22;29(29):e202300008. doi: 10.1002/chem.202300008. Epub 2023 Apr 14. Chemistry. 2023. PMID: 36786481 Free PMC article. Review.
-
Strain-Release Driven Epoxidation and Aziridination of Bicyclo[1.1.0]butanes via Palladium Catalyzed σ-Bond Nucleopalladation.Angew Chem Int Ed Engl. 2023 Feb 6;62(7):e202217064. doi: 10.1002/anie.202217064. Epub 2023 Jan 12. Angew Chem Int Ed Engl. 2023. PMID: 36507714 Free PMC article.
-
Strain-Release Driven Epoxidation and Aziridination of Bicyclo[1.1.0]butanes via Palladium Catalyzed σ-Bond Nucleopalladation.Angew Chem Weinheim Bergstr Ger. 2023 Feb 6;135(7):e202217064. doi: 10.1002/ange.202217064. Epub 2023 Jan 12. Angew Chem Weinheim Bergstr Ger. 2023. PMID: 38516047 Free PMC article.
-
Fused-Linked and Spiro-Linked N-Containing Heterocycles.Int J Mol Sci. 2025 Aug 1;26(15):7435. doi: 10.3390/ijms26157435. Int J Mol Sci. 2025. PMID: 40806564 Free PMC article. Review.
References
-
- None
-
- Rice K. D., Aay N., Anand N. K., Blazey C. M., Bowles O. J., Bussenius J., Costanzo S., Curtis J. K., Defina S. C., Dubenko L., Engst S., Joshi A. A., Kennedy A. R., Kim A. I., Koltun E. S., Lougheed J. C., Manalo G.-C. L., Martini J.-F., Nuss J. M., Peto C. J., Tsang T. H., Yu P., Johnston S., ACS Med. Chem. Lett. 2012, 3, 416–421; - PMC - PubMed
-
- Johansson A., Löfberg C., Antonsson M., von Unge S., Hayes M. A., Judkins R., Ploj K., Benthem L., Lindén D., Brodin P., Wennerberg M., Fredenwall M., Li L., Persson J., Bergman R., Pettersen A., Gennemark P., Hogner A., J. Med. Chem. 2016, 59, 2497–2511; - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

