Chemoprotective Antimalarial Activity of P218 against Plasmodium falciparum: A Randomized, Placebo-Controlled Volunteer Infection Study
- PMID: 33556040
- PMCID: PMC8045640
- DOI: 10.4269/ajtmh.20-1165
Chemoprotective Antimalarial Activity of P218 against Plasmodium falciparum: A Randomized, Placebo-Controlled Volunteer Infection Study
Abstract
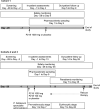
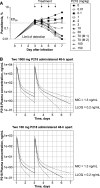
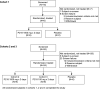
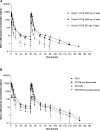
P218 is a highly selective dihydrofolate reductase inhibitor with potent in vitro activity against pyrimethamine-resistant Plasmodium falciparum. This single-center, randomized, double-blind, placebo-controlled phase Ib study evaluated P218 safety, pharmacokinetics, and chemoprotective efficacy in a P. falciparum sporozoite (PfSPZ) volunteer infection study (VIS). Consecutive dose safety and tolerability were evaluated (cohort 1), with participants receiving two oral doses of P218 1,000 mg 48 hours apart (n = 6), or placebo (n = 2). P218 chemoprotective efficacy was assessed (cohorts 2 and 3) with direct venous inoculation of 3,200 aseptic, cryopreserved PfSPZ (NF54 strain) followed 2 hours later with two P218 doses of 1,000 mg (cohort 2, n = 9) or 100 mg (cohort 3, n = 9) administered 48 hours apart, or placebo (n = 6). Parasitemia was assessed from day 7 using quantitative PCR targeting the var gene acidic terminal sequence (varATS qPCR). By day 28, all participants in cohort 2 (P218 1,000 mg) and 8/9 in cohort 3 (P218 100 mg) were sterilely protected post-PfSPZ VIS, confirming P218 P. falciparum chemoprotective activity. With placebo, all six participants became parasitemic (geometric mean time to positive parasitemia 10.6 days [90% CI: 9.9-11.4]). P218 pharmacokinetics were similar in participants with or without induced infection. Adverse events of any cause occurred in 45.8% (11/24) of participants who received P218 and 50.0% (4/8) following placebo; all were mild/moderate in severity, transient, and self-limiting. There were no clinically relevant changes in laboratory parameters, vital signs, or electrocardiograms. P218 displayed excellent chemoprotective efficacy against P. falciparum with favorable safety and tolerability.
Conflict of interest statement
Disclosures: M. F. C., M. E. G., C. D., B. C., M. H. C.-R., and S. C. are employees of Medicines for Malaria Venture. P.-J. B. and A. L. are employees of SGS Life Sciences. M. W. M. is an employee of ICON Clinical Research GmbH. I. A.-B. was an employee of GlaxoSmithKline at the time of the study and is currently employed by the Art of Discovery. S. V. is an employee of GlaxoSmithKline. G. L. is an employee of PTx Solutions. A. R.-U. is an employee of the Institute of Tropical Medicine, Antwerp. J.-P. V. G. is an employee of the University of Antwerp. SGS Life Sciences, ICON plc, PTx Solutions, the Institute of Tropical Medicine, and the University of Antwerp, all received support from Medicines for Malaria Venture related to the conduct of this study. Naomi Richardson of Magenta Communications Ltd wrote the first draft of this article from the statistical output, provided editorial support, collated author contributions, and provided graphic services. Named authors employed by Medicines for Malaria Venture were involved in the design, execution, analysis, interpretation, and reporting of the study as detailed in the author contributions.
Figures

References
-
- WHO , 2019. World Malaria Report. Geneva, Switzerland: World Health Organization. Available at: https://www.who.int/publications-detail/world-malaria-report-2019. Accessed 31 July, 2020.
-
- Stevens GA, Finucane MM, De-Regil LM, Paciorek CJ, Flaxman SR, Branca F, Pena-Rosas JP, Bhutta ZA, Ezzati M; Nutrition Impact Model Study Group , 2013. Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995–2011: a systematic analysis of population-representative data. Lancet Glob Health 1: e16–e25. - PMC - PubMed
-
- WHO Global Malaria Programme, WHO Department of Reproductive Health and Research, WHO Department of Maternal N, Child and Adolescent Health , 2013. WHO Policy Brief for the Implementation of Intermittent Preventive Treatment of Malaria in Pregnancy Using Sulfadoxine-Pyrimethamine (IPTp-SP). Geneva, Switzerland: World Health Organization. Available at: https://www.who.int/malaria/publications/atoz/iptp-sp-updated-policy-bri.... Accessed July 31, 2020.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

