Treatment sequences of patients with advanced colorectal cancer and use of second-line FOLFIRI with antiangiogenic drugs in Japan: A retrospective observational study using an administrative database
- PMID: 33556095
- PMCID: PMC7870079
- DOI: 10.1371/journal.pone.0246160
Treatment sequences of patients with advanced colorectal cancer and use of second-line FOLFIRI with antiangiogenic drugs in Japan: A retrospective observational study using an administrative database
Abstract
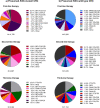
The objectives were to describe treatment sequences for advanced colorectal cancer (CRC), use of second-line FOLFIRI (leucovorin, 5-fluorouracil, irinotecan) plus antiangiogenic drug (bevacizumab, ramucirumab, aflibercept beta) therapy, and the factors associated with the duration of antitumor drug treatment from second-line antiangiogenic therapy in Japan. This retrospective observational study was conducted using a Japanese hospital-based administrative database. Patients were enrolled if they started adjuvant therapy (and presumably experienced early recurrence) or first-line treatment for advanced CRC between May 2016 and July 2019, and were analysed until September 2019. Factors associated with overall treatment duration from second-line treatment with FOLFIRI plus antiangiogenic drugs were explored with multivariate Cox regression analysis. The most common first-line treatments were FOLFOX (leucovorin, 5-fluorouracil, oxaliplatin) or CAPOX (capecitabine, oxaliplatin) with bevacizumab (presumed RAS-mutant CRC) and FOLFOX with panitumumab (presumed RAS-wild type CRC). The most common second-line treatments were FOLFIRI-based. Many patients did not transition to subsequent lines of therapy. For second-line treatment, antiangiogenic drugs were prescribed more often for patients with presumed RAS-mutant CRC, right-sided CRC, and independent activities of daily living (ADL). The median duration of second-line FOLFIRI plus antiangiogenic drug treatment was 4.5 months; 66.2% of patients transitioned to third-line therapy. Low body mass index and not fully independent ADL were significantly associated with shorter overall duration of antitumor drug treatment from second-line therapy. Left-sided CRC, presumed RAS-wild type CRC, previous use of oral fluoropyrimidines and use of proteinuria qualitative tests, antihypertensives, or anticholinergics during second-line therapy were significantly associated with longer treatment. Treatment of advanced CRC in Japan is consistent with both international and Japanese guidelines, but transition rates to subsequent therapies need improvement. In addition to antitumor drug treatment, better ADL, higher body mass index, management of hypertension, and proteinuria tests were associated with continuation of sequential therapy that included antiangiogenic drugs.
Conflict of interest statement
ES has received honoraria from Taiho Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd., Eli Lilly Japan Co., Ltd., Merck Bio Pharma Co., Ltd., Sanofi Co., Ltd., and Yakult Honsha Co., Ltd. YK has received honoraria from Bayer Co., Ltd., Chugai Pharmaceutical Co., Ltd., Yakult Honsha Co., Ltd., Sanofi Co., Ltd., Eli Lilly Japan Co., Ltd., Taiho Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., and Merck Co., Ltd. AM has received honoraria from Eli Lilly Japan Co., Ltd., Chugai Pharmaceutical Co., Ltd., and Takeda Pharmaceutical Co., Ltd. HS has received research funding from Ono Pharmaceutical Co., Ltd., Taiho Pharmaceutical Co., Ltd., and Takeda Pharmaceutical Co., Ltd., and honoraria from Bayer Co., Ltd., Bristol-Myers Squibb Co., Ltd., Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., Eli Lilly Japan Co., Ltd., Merck Bio Pharma Co., Ltd., MSD Co., Ltd., Ono Pharmaceutical Co., Ltd., Sanofi Co., Ltd., Taiho Pharmaceutical Co., Ltd., Takeda Co., Ltd., and Yakult Honsha Co., Ltd. This study was sponsored by Eli Lilly Japan K.K., manufacturer/licensee of ramucirumab. YT, ZC, and YP are employees and/or minor shareholders of Eli Lilly Japan K.K. Medical writing assistance provided by Serina Stretton, PhD, CMPP, and Rebecca Lew, PhD, CMPP, of ProScribe – Envision Pharma Group was funded by Eli Lilly Japan K.K. This does not alter our adherence to PLOS ONE policies on sharing data and materials, but the data used for this study are owned by a third party (Medical Data Vision Co. Ltd.).
Figures

References
-
- Ministry of Health, Labour and Welfare. Overview of the National Cancer Registry 2016. Available from: www.mhlw.go.jp/content/10900000/000468976.pdf.
-
- Ministry of Health, Labour and Welfare. Cancer Information Service, National Cancer Center, Japan. Cancer Registry and Statistics. Vital Statistics of Japan. Available from: https://ganjoho.jp/reg_stat/statistics/dl/index.html#mortality.
-
- Tabernero J, Yoshino T, Cohn AL, Obermannova R, Bodoky G, Garcia-Carbonero R, et al. Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): a randomised, double-blind, multicentre, phase 3 study. Lancet Oncol. 2015;16(5):499–508. 10.1016/S1470-2045(15)70127-0 - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

